What do you mean by electron gain enthalpy
Electron gain enthalpy is sometimes also referred to as Electron affinity although there is a minute difference between them.
To define electron gain enthalpy , sometimes, it is also called Electron affinity , although there exists a small difference between them. The amount of energy released when an electron is added to an isolated gaseous atom is characterised as an electron gain enthalpy. During the addition of the electron, either the energy can be released or absorbed. Let us consider two metals , Sodium and Magnesium. Metals will lose electrons to obtain the inert gas configuration.
What do you mean by electron gain enthalpy
How many of you are aware of what electrons are? But, what is electron gain enthalpy? Well, not anymore! In this chapter, we will look at the concept of electron gain enthalpy and discuss it in greater detail. Electron gain enthalpy of an element is the energy released when a neutral isolated gaseous atom accepts an extra electron to form the gaseous negative Ion i. Greater the amount of energy released in the above process , higher is the electron gain enthalpy of the element. The electron gain enthalpy of an element is a measure of the firmness or strength with which an extra electron is bound to it. It is measured in electron volts per atom or kJ per mole. It can be an endothermic or exothermic reaction when you add an electron to the atom. As the size of the atom increases, the distance between the nucleus and the last shell which receives the incoming electrons increases. This decreases the force of attraction between the nucleus and the incoming electron.
Mainly on atomic radius and the nuclear charge. From moving top to bottom the electron gain enthalpy becomes less negative and more negative from moving left to right in a period of the periodic table.
Electron gain enthalpy is mainly described or defined as an element releases energy when a neutral isolated gaseous atom that always accepts an extra electron to develop the gaseous negative ion which is the anion. We can conclude the measure of the electron gain enthalpy of an element by its particular firmness or strength with which an extra electron is always bound to it. The unit by which the electron gains enthalpy of an electron is measured in electron volts per atom or kJ per mole. Either exothermic or endothermic is the usual process of adding an electron to the atom. However, the electron gain enthalpy is negative when energy is released due to the addition of an electron in an atom.
Electron gain enthalpy is often confused with electron affinity but to make it simpler to understand we can say that it is the energy that is released when a neutral isolated gaseous atom accepts an extra electron and forms a gaseous negative ion, called an anion. It is a measure of the strength with which an extra electron is bound to the element. The greater the amount of energy released in the reaction, the higher is the electron gain enthalpy of the element. Electron gain enthalpy is measured in electron volts per atom or kJ per mole. Generally, there is a release of energy when an electron is added to an atom, and the electron gain enthalpy for such elements is negative.
What do you mean by electron gain enthalpy
The reaction can be given as below:. On the basis of the nature of the element, the process of accepting electrons in an atom can either be exothermic or endothermic. In general, energy is released when an electron is added to an atom and the electron gain enthalpy for such elements is negative. The electron gain enthalpy of halogens is highly negative because it needs only one electron to achieve the nearest noble gas configuration. And for noble gases, it is highly positive because the extra electron has to be placed in the next higher principal quantum level which requires lots of energy. When one electron is added to an atom, it becomes negatively charged ions. Now if we add the second electron to this negatively charged ion, it experiences repulsive forces due to the electrons already present in that shell. Additional energy should be provided to overcome these repulsive forces.
Rubius diablo 4
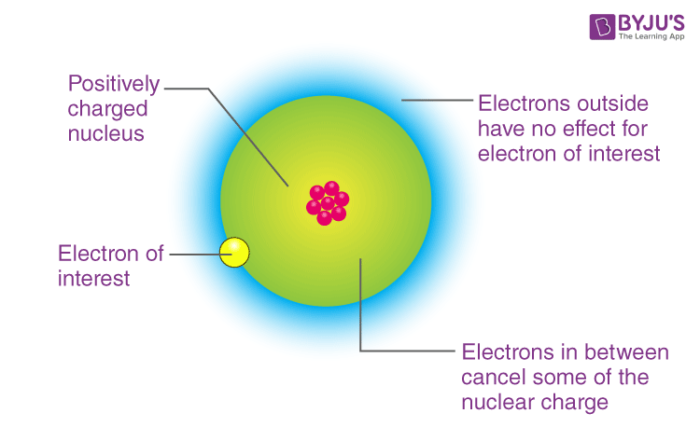
The nuclear charge shielding effect also describes the easy removal of the valence shell or outermost shell electrons from the atom, the atomic size is also explained by this effect. As we move from the direction of chlorine to iodine , the electron gain enthalpies become very negative due to the corresponding increase in their atomic radii. Mainly on atomic radius and the nuclear charge. The electron-electron repulsion in the relatively compact to the 2p subshell is comparably strong due to its tiny size. Negative Electron Gain Enthalpy: Negative electron gain enthalpy indicates negative values when the energy gets released. Hence, it will remove the maximum amount of energy. Therefore, the electron gain enthalpy of the chlorine compound is more negative as compared to the Sulphur. Positive Electron Gain Enthalpy: In positive electron gain enthalpy, the element is reluctant to accept the other electron. Related links. Share via. All of this results in the positive charge of the second electron gain enthalpy.
Electron gain enthalpy is sometimes also referred to as Electron affinity although there is a minute difference between them. Electron gain enthalpy is defined as the amount of energy released when an electron is added to an isolated gaseous atom.
Electron gain enthalpy represents the heat given off by the ionization reaction, which means that it will carry a negative sign. The electron-electron repulsion in the relatively compact to the 2p subshell is comparably strong due to its tiny size. Related articles. Hence, their electron gain enthalpy has large positive values. The exception to this rule are the noble gases, which have very low electron affinities because their outermost shells are already full of electrons. Electron gain enthalpy is mainly described or defined as an element releases energy when a neutral isolated gaseous atom that always accepts an extra electron to develop the gaseous negative ion which is the anion. All of this results in the positive charge of the second electron gain enthalpy. Subatomic Particles. Download the App Watch lectures, practise questions and take tests on the go. Why noble gases have zero electron gain enthalpy? As a result, atoms with more positive nuclear charges tend to have higher electron affinities. At last we will discuss some important questions related to zwitterion.

You are absolutely right. In it something is also to me it seems it is excellent idea. I agree with you.
In it something is also to me your idea is pleasant. I suggest to take out for the general discussion.
I apologise, I too would like to express the opinion.