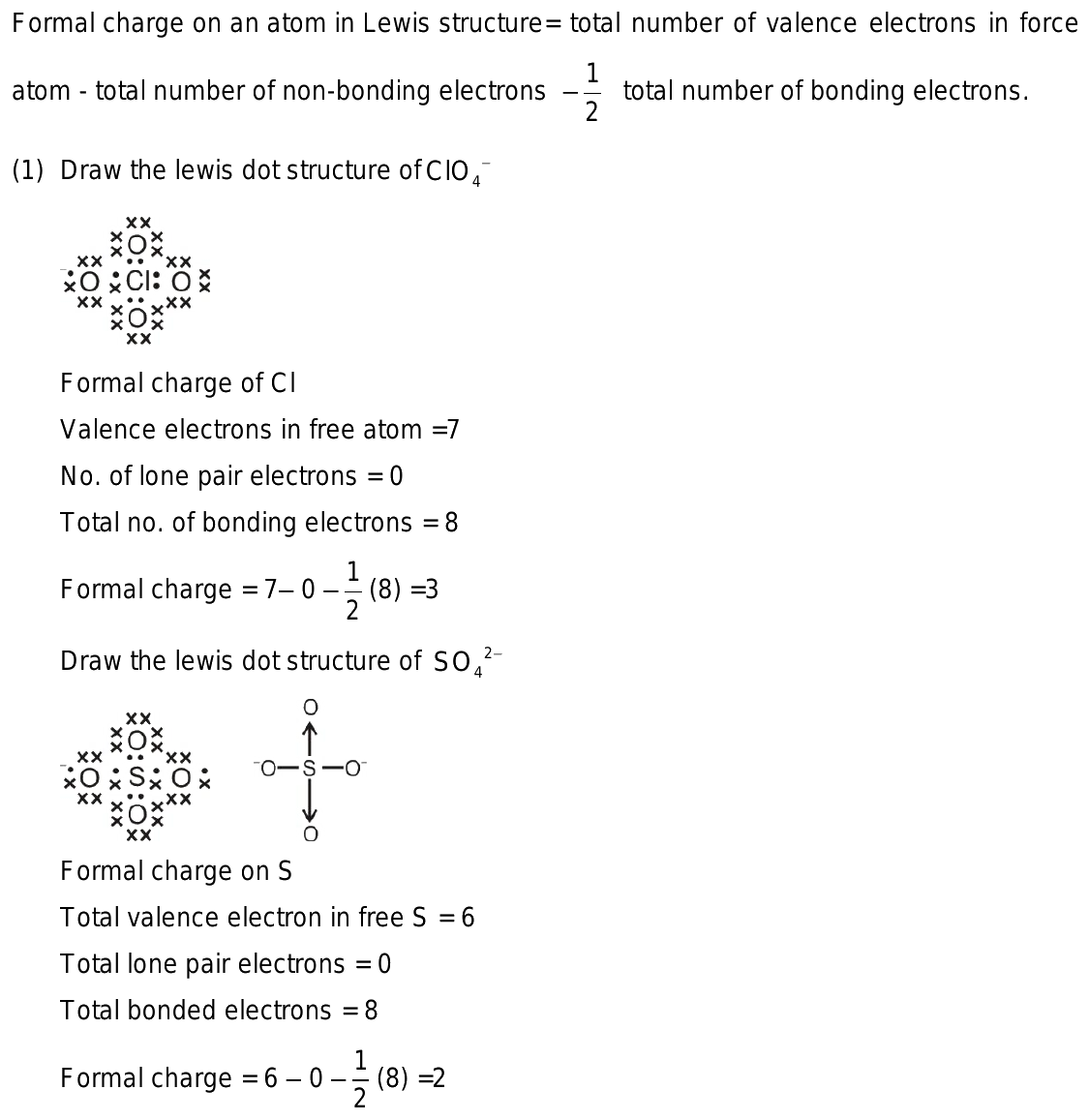
So4 2 formal charge
What is the formal charge on Cl in the following lewis structure. Calculate the formal charge on Cl atom in H C l O 4. Octet is completed in which of the following?
Lewis structures are another way to represent molecules. Lewis Structures were introduced by Gilbert N. Lewis in Lewis suggested the use of lines between atoms to indicate bonds, and pairs of dots around atoms to indicate lone or non-bonding pairs of electrons. In the example above, 3 hydrogen atoms with one valence electron each form three bonds with one nitrogen atom with 5 valence electrons.
So4 2 formal charge
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. First, we have to find out how many valence electrons are in the molecule. Then, draw a skeletal molecule in which the central atom forms a single bond with each of the other atoms. The least electronegative element will make up the center atom. Now, fill the octet of the most electronegative element and then the other elements. Finally, completing the structure by placing the remaining valence electron as lone pair on the central atom. Sulfur is the central atom and four oxygen atoms are located around the sulfur atom Adding electron pair between Sulfur and oxygen to represent a chemical bond. Now, completing octet of Oxygen i.
Bibcode : ClCh In the case of sulfate or sulfite we do not need to make double bonds, but another example in which a double bond would be necessary is C 2 H 4. Double and even triple bonds are formed so4 2 formal charge there is a lack of electrons needed to make full octets for all of those atoms that need them.
Three cases can be constructed that do not follow the octet rule, and as such, they are known as the exceptions to the octet rule. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. However, it is hard to imagine that one rule could be followed by all molecules. There is always an exception, and in this case, three exceptions:. The first exception to the Octet Rule is when there are an odd number of valence electrons.
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of We can easily prepare sulfates via oxidizing metal sulfites and sulfides. We can also use sulfuric acid and metals to get our desired sulfate salts. Since we can easily get hold of this ion, be it naturally or synthetically, this helps us in our daily lives in a lot more ways than you can think of right now! From body and hygiene-care products like toothpaste, shampoos, soaps, and detergents to water treatment procedures, we can find the application of sulfate compounds everywhere. It plays an important factor in acid rain composition. Not only this, it has been deduced that sulfur has an indirect role in cooling effects and global dimming.
So4 2 formal charge
The SO4 2- Sulfate Ion , comprised of one sulfur atom and four oxygen atoms, presents a captivating example of a chemical species with intriguing properties. At the heart of comprehending the characteristics and reactivity of SO4 2- lies the exploration of its Lewis structure. Determine Total Valence Electrons. To begin, identify the valence electrons of each atom in the SO4 2- molecule. Sulfur S belongs to Group 16, contributing 6 valence electrons, while each oxygen O atom in Group 16 contributes 6 valence electrons. In the SO4 2- ion, sulfur S is the central atom due to its lower electronegativity compared to oxygen O. Establish single bonds electron pairs between sulfur S and each oxygen O atom.
H dale funeral directors
The metal—oxygen bonds in sulfate complexes can have significant covalent character. Of course, there are, some exceptions: very small atoms H, Be and B have less than an octet, and some main group atoms in the third period and below P, S, Cl, Br, and I may have more than an octet but most elements still strive for the completion of their outer valence shell with 8 electrons. Photoelectric Effect. Subtract the number of bonding electrons from the total number of valence electrons. Let's take a look at another incomplete octet situation dealing with boron, BF 3 Boron trifluorine. Significant Figures. Elements A has one electron in its valence shell and its principal qua Group 1A and 2A Reactions. Email Link. Although written with simple anhydrous formulas, these conversions generally are conducted in the presence of water. Maxwell-Boltzmann Distribution. Q: Consider the three possible structures of the thiocyanate ion SCN'. We'll put the Sulfur in the center, and then the four Oxygens will go on the outside. This exemplifies the fact that incomplete octets are rare, and other configurations are typically more favorable, including bonding with additional ions as in the case of BF 3. By forming three bonds, nitrogen gains 3 electrons to make a total of 8 surrounding it.
SO is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more.
The two oxygens with the single bonds to sulfur have seven electrons around them in this structure six from the three lone pairs and one from the bond to sulfur. Q: For which one or ones of the following molecules or ions would we expect to use resonance forms in… A: For which one or ones of the following molecules or ions would we expect to use resonance forms in…. Then we would…. Periodic Trend: Atomic Radius. Speed of Light. Acids Introduction. Each atom has a perfect octet, right? Average Rate of Reaction. Henderson-Hasselbalch Equation. Now, fill the octet of the most electronegative element and then the other elements. So for Sulfur, 6 minus zero, there are no nonbonding; and now we have 2, 4, 6, 8, 10, 12 total bonding electrons. The Quadratic Formula. Bibcode : ESD Step 3. Sulfates occur widely in everyday life.

I think, that you are not right. I am assured. I can prove it.
You are not right. I can defend the position. Write to me in PM.