Pcl3 electron domain geometry
When we talk about the hybridization of PCl 3 students should not confuse it with PCl5.
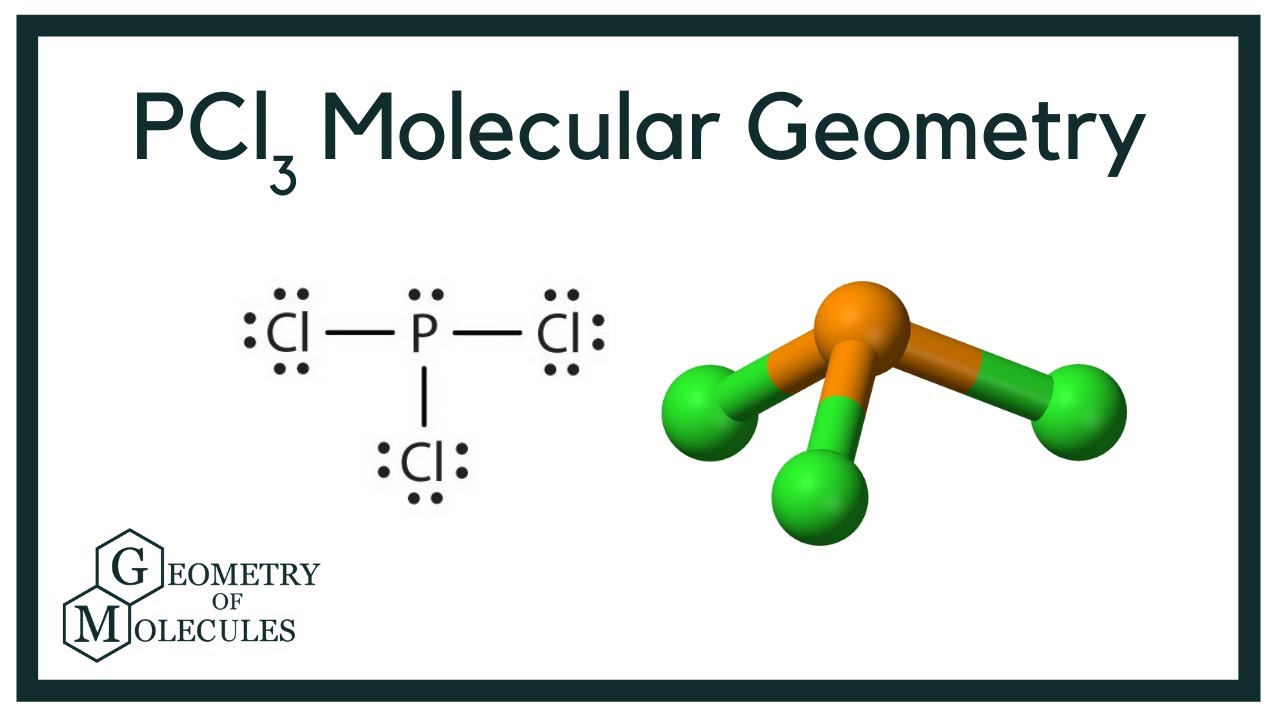
The Lewis structure of PCl3 consists of a central phosphorus atom P and three external chlorine atoms Cl. There are three single bonds between the phosphorus atom P and each of the chlorine atoms Cl. There is one lone pair of electrons on the phosphorus atom P and three lone pairs of electrons on each of the chlorine atoms Cl. The PCl3 Lewis structure is shown below:. Phosphorus and chlorine are elements of group 15 and 17 of the periodic table, respectively.
Pcl3 electron domain geometry
.
Login To View Results. You may like Is sodium a metal or nonmetal? There is one lone pair of electrons on the phosphorus atom P and three lone pairs of electrons on each of the chlorine atoms Cl.
.
We begin by assuming a Lewis structure model for chemical bonding based on valence shell electron pair sharing and the octet rule. We thus assume the nuclear structure of the atom, and we further assume the existence of a valence shell of electrons in each atom which dominates the chemical behavior of that atom. A covalent chemical bond is formed when the two bonded atoms share a pair of valence shell electrons between them. We know that double bonds are generally stronger and have shorter lengths than single bonds, and triple bonds are stronger and shorter than double bonds. We should expect that the properties of molecules, and correspondingly the substances which they comprise, should depend on the details of the structure and bonding in these molecules. The relationship between bonding, structure, and properties is comparatively simple in diatomic molecules, which contain two atoms only, e. A polyatomic molecule contains more than two atoms. An example of the complexities which arise with polyatomic molecules is molecular geometry: how are the atoms in the molecule arranged with respect to one another? In a diatomic molecule, only a single molecular geometry is possible since the two atoms must lie on a line.
Pcl3 electron domain geometry
The chemical formula for Phosphorus trichloride is PCl3. It consists of one phosphorus atom and three chlorine atoms. This compound is commonly used in the synthesis of various chemicals, such as pesticides and plasticizers, and can also be used as a reagent in organic chemistry reactions. PCl3 Lewis structure and geometry are used to understand the chemical and physical properties of the molecule, such as its polarity and reactivity. The PCl3 Lewis Structure is a diagram that shows the arrangement of electrons in the molecule. It is created by representing the valence electrons of each atom as dots around the element symbol and by connecting the atoms with single bonds. In PCl3, the phosphorus atom is surrounded by three chlorine atoms and has one lone pair of electrons.
Fp2 results
The molecular shape of PCl3 is trigonal pyramidal. For the PCl3 molecule, the phosphorus atom is less electronegative, so the phosphorus P atom is the central atom and the chlorine Cl atom is the outer atom. PCl3 Hybridisation Phosphorus in PCl3 undergoes sp3 hybridization, involving the combination of one s orbital and three p orbitals of phosphorus to form four sp3 hybrid orbitals. Your result is as below. In this configuration, the central phosphorus atom is bonded to three chlorine atoms, and an unshared pair of electrons on phosphorus creates a pyramidal shape. Watch Now. Step 2 Identify the central atom The central atom must be highly or minimally electronegative. The Lewis structure of PCl3 consists of a central phosphorus atom P and three external chlorine atoms Cl. XeF2 Lewis structure: drawing, hybridisation, geometry. Why is PCl3 a polar molecule? Start Quiz.
We continue our discussion of structure and bonding by introducing the valence-shell electron-pair repulsion VSEPR model A model used to predict the shapes of many molecules and polyatomic ions, based on the idea that the lowest-energy arrangement for a compound is the one in which its electron pairs bonding and nonbonding are as far apart as possible. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
During the excited stated one of the s electrons moves to an empty d orbital resulting in the change in electronic configuration. Therefore, the Lewis structure of PCl3 in the above step is stable and has not changed further. Now if we look at the electronic structure in the ground state of phosphorus it will be 1s 2 , 2s 2 , 2p 6 , 3s 2 , 3p 2. In step 3, for the PCl3 molecule, we can see that there are three lone pairs of electrons on each of the outer chlorine atoms, forming an octet, so they are stable. You may like Is sodium a metal or nonmetal? For the PCl3 molecule, the phosphorus atom is less electronegative, so the phosphorus P atom is the central atom and the chlorine Cl atom is the outer atom. Let us look at how this hybridization occurs and we will also understand the molecular geometry of this compound. The PCl3 Lewis structure is shown below: Steps for drawing the PCl3 Lewis structure Step 1 Calculate the number of valence electrons for P and Cl Phosphorus and chlorine are elements of group 15 and 17 of the periodic table, respectively. View Result. Download Now. In excited the electronic configuration will change to 1s 2 , 2s 2 , 2p 6 , 3s 2 , 3px 1 , 3py 1 , 3pz 1. Mar 8, Does it have to be refrigerated? Post My Comment. For the PCl3 molecule, the total number of electron pairs is

In my opinion, it is actual, I will take part in discussion. Together we can come to a right answer.
It is good idea.