Oxalic acid dihydrate pka
JavaScript seems to be disabled in your browser.
Title: Oxalic Acid. CAS Registry Number: CAS Name: Ethanedioic acid. Molecular Formula: C 2 H 2 O 4. Molecular Weight: Percent Composition: C Literature References: Present in many plants and vegetables, notably in those of the Oxalis and Rumex families, where it occurs in the cell sap of the plant as the potassium or calcium salt.
Oxalic acid dihydrate pka
Choose a language and region. United States. This section provides an overview for oxalic acid dihydrate as well as their applications and principles. Also, please take a look at the list of 16 oxalic acid dihydrate manufacturers and their company rankings. Here are the top-ranked oxalic acid dihydrate companies as of March, 1. Hemadri Chemicals, 2. Central Drug House, 3. The CAS number is It has a molecular weight of It is a white crystal or crystalline powder at room temperature. The compound is soluble in water and ethanol and insoluble in diethyl ether. Oxalic acid dihydrate is hygroscopic, and therefore, when left in moist air, it will assume the dihydrate state. Dihydrate also precipitates from aqueous solutions.
Oxalic acid is prepared in the laboratory by oxidizing sucrose with Nitric acid. JavaScript seems to be disabled in your browser.
With an accout for my. Oxalic acid is the chemical compound with the formula H 2 C 2 O 4. It is a relatively strong organic acid , being about 10, times stronger than acetic acid. The di-anion, known as oxalate, is also a reducing agent as well as a ligand in coordination chemistry. Many metal ions form insoluble precipitates with oxalate, a prominent example being calcium oxalate, which is the primary constituent of the most common kind of kidney stone. Although it can be readily purchased, oxalic acid can be prepared in the laboratory by oxidizing sucrose using nitric acid in the presence of a small amount of vanadium pentoxide as a catalyst. Typically oxalic acid is obtained as the dihydrate.
It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis , commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid has much greater acid strength than acetic acid. The preparation of salts of oxalic acid crab acid [ citation needed ] from plants had been known, at least since , when the Dutch botanist and physician Herman Boerhaave isolated a salt from wood sorrel , akin to kraft process. By , Scheele had shown that "sugar acid" and oxalic acid from natural sources were identical. Oxalic acid is mainly manufactured by the oxidation of carbohydrates or glucose using nitric acid or air in the presence of vanadium pentoxide.
Oxalic acid dihydrate pka
Q: buffer solution is prepared by dissolving 4. A: The solution is as follows:. Q: A chemistry graduate student is given A: The Henderson-Hasselbalch equation gives the pH of buffer solution it is mathematically represented….
Reality kings xvideos
In one pathway, oxaloacetate , a component of the Krebs citric acid cycle , is hydrolyzed to oxalate and acetic acid by the enzyme oxaloacetase : [36]. If Oxalic acid is spilled, consider the following steps: Evacuate every person on the premises and control the entrance area. Molecular Plant Pathology. The company serves the life science industry and has a broad portfolio of , products, including biopharmaceutical manufacturing, industrial microbiology, reagent, life science research, and water purification products. The company has certified quality management and has acquired several certifications including ISO for risk management criteria and WHO GSDP for preserved quality of pharmaceutical products and good distributive practices. Oxalic acid is applied to lighten the color and soften the tone of wood and leather. Sal nativum plantarum paratus de succo illarum recens presso. The chemical products have a wide range of applications and can be used in pathological examination, as food additives, for analysis, and in the pharmaceutical industry. It is a divalent carboxylic acid with two carboxyl groups and acts as an ionized acid in an aqueous solution. CAUTION: Potential symptoms of overexposure by ingestion are corrosion of alimentary tract mucosa; localized pain; vomiting, shock, hypotension, cardiovascular collapse; headache, muscle cramps, tatany; convulsions, stupor, coma; kidney damage. Potassium Iodide. Citrus juice contains small amounts of oxalic acid. Small amounts of oxalic acid enhances plant resistance to fungi, but higher amounts cause widespread programmed cell death of the plant and help with fungi infection.
Submitted by Melissa H.
United States Department of Agriculture. Workers should remove and wash contaminated clothing after handling it. Fastening Components. Oxalic acid is harmful to humans. Skin: Rashes, pain, redness, skin burns. Archived from the original PDF on The supernatant sample is discarded, and the oxalate precipitate is collected for determining rare metals. October Testing the pH: The best procedure is to add the baking soda until the solution reaches a pH of 7. The Journal of Physical Chemistry A. Clarke, A. Ingestion: Rinse your mouth with plenty of water. January

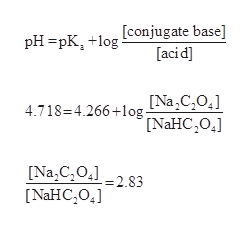
Really and as I have not realized earlier