Nitrogen trifluoride lewis structure
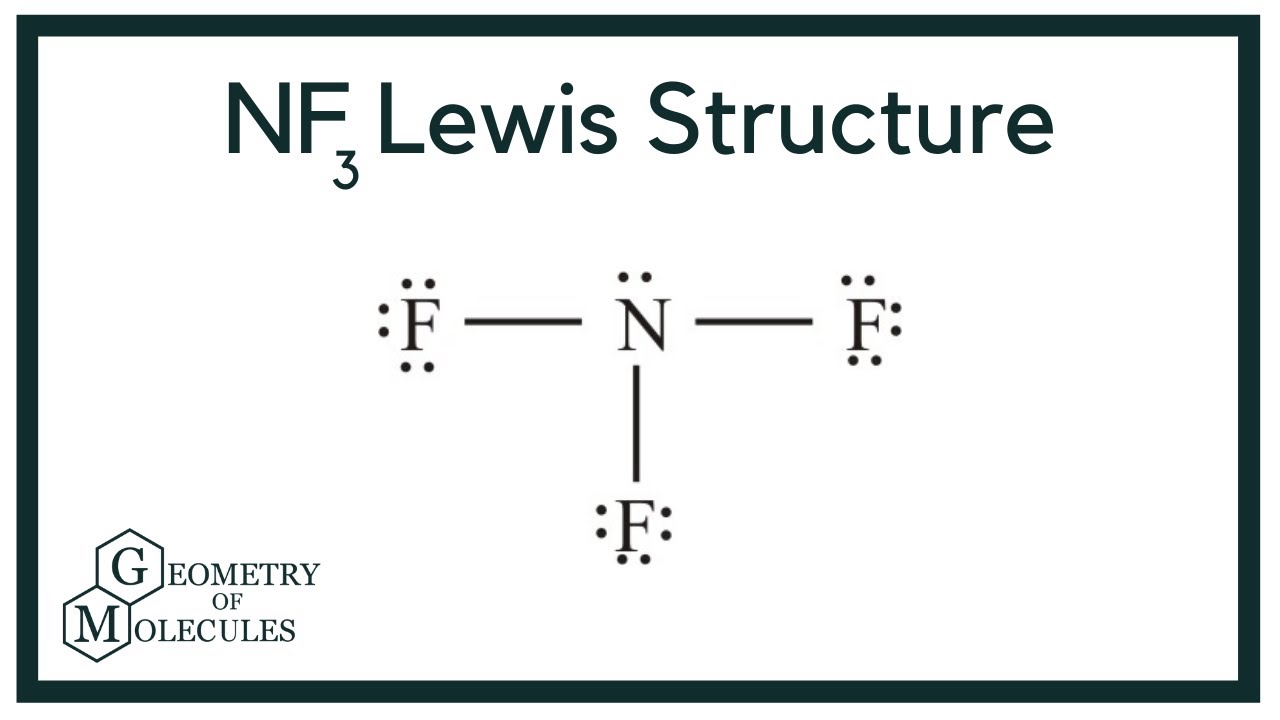
The Lewis structure of nitrogen trifluoride NF 3 consists of three N-F bonds and a lone pair on the nitrogen atom, while each fluorine atom has three lone pairs. Drawing the Lewis structure of NF 3 involves several nitrogen trifluoride lewis structure starting from the valence electrons of nitrogen and fluorine atoms, which are explained in detail in this tutorial.
Nitrogen trifluoride NF3 is a colorless, nonflammable gas with routine usage in the microelectronics industry. It is an essential molecule in plasma science as an efficient fluorine source in manufacturing massive-scale integrated circuits. Although NF3 is indispensable in the electronics industry, it is a significant greenhouse gas, and its heat storage capacity is 17, times that of carbon dioxide. The molecule is a hazardous greenhouse gas that can persist in the atmosphere for years. In , it was included in the list of controlled gases under the United Nations Framework Convention on Climate Change[]. Fluorine is a group VIIA element and has seven electrons in its last shell valence shell. Nitrogen is a group VA element in the periodic table and contains five electrons in its last shell.
Nitrogen trifluoride lewis structure
The NF3 molecule, composed of one nitrogen atom and three fluorine atoms, holds within its structure a fascinating arrangement of atoms and electrons that govern its chemical behavior. By delving into the principles of valence electrons, formal charges, and the octet rule, we can decipher the molecular puzzle that NF3 presents. Determine Total Valence Electrons. Begin by identifying the number of valence electrons for each atom in the NF3 molecule. Nitrogen N is in Group 15 of the periodic table and has 5 valence electrons, while Fluorine F is in Group 17 and possesses 7 valence electrons. Choose the Central Atom. In the NF3 molecule, nitrogen N is the least electronegative atom. As a result, it will serve as the central atom, with fluorine F atoms surrounding it. Connect Atoms with Electron Pairs. Connect the nitrogen N atom to each fluorine F atom with a single bond a pair of electrons.
What is a formal charge, and how do I calculate it in the NF3 Lewis structure? Distribute the remaining valence electrons around the central nitrogen N atom as lone pairs, following the octet rule.
In the lewis structure of Nitrogen trifluoride NF 3 , there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Each step of drawing the lewis structure of NF 3 is explained in detail in this tutorial. After drawing the lewis structure of NF 3 , you can decide shape of the NF 3 molecule. In the lewis structure of NF 3 , there are three N-F bonds and one lone pair on nitrogen atom which is the center atom. You have to follow few steps to draw the lewis structure of NF 3.
In the lewis structure of Nitrogen trifluoride NF 3 , there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Each step of drawing the lewis structure of NF 3 is explained in detail in this tutorial. After drawing the lewis structure of NF 3 , you can decide shape of the NF 3 molecule. In the lewis structure of NF 3 , there are three N-F bonds and one lone pair on nitrogen atom which is the center atom.
Nitrogen trifluoride lewis structure
Transcript: Hi, this is Dr. We're going to do the Lewis structure for NF3, nitrogen trifluoride. On the periodic table, Nitrogen is in group 5 or 15, so it has 5 valence electrons; and then Fluorine is in group 7 or 17, it has 7. We've got three Fluorines, though, so let's multiply that by 3. That equals, 21 plus 5, is 26 valence electrons. Nitrogen is the least electronegative, so that's going to go in the center here, and then we'll put the F's, those Fluorines around there, and we have three of them. OK, so at this point we've got 26 valence electrons to work with. Let's first put them between atoms to form that chemical bond there. So 2, 4, 6; and spread them around the outside now.
Spanish words filipino
Its Lewis structure provides a visual representation of its molecular arrangement and electron distribution, aiding in understanding its chemical properties and interactions. For NF 3 , there are three fluorine atoms and one nitrogen atom. Because nitrogen trifluoride is a simple molecule, these steps are not complex and do not require all steps which are used to draw lewis structures of complex molecules and ions. Formal charge is a measure of the electron distribution in a molecule. Distribute the remaining valence electrons around the central nitrogen N atom as lone pairs, following the octet rule. Step 2: Select the central atom It is essential to consider both the ability to have a greater valence and to be the most electropositive element. The NF3 molecule, composed of one nitrogen atom and three fluorine atoms, holds within its structure a fascinating arrangement of atoms and electrons that govern its chemical behavior. Remember that, there are total of thirteen electron pairs. The polarity of a molecule is determined by the electronegativity difference between the atoms and the molecular geometry. These steps include: determining the total number of electrons in the valence shells of the nitrogen and fluorine atoms identifying the total number of electron pairs as lone pairs and bonds selecting the central atom marking the lone pairs on the atoms marking any charges on the atoms Finally, the stability of the structure needs to be checked, and any charges on the atoms minimized by converting lone pairs to bonds to obtain the best Lewis structure. This is because nitrogen has five valence electrons, and it forms three covalent bonds with fluorine atoms, leaving one pair of electrons unshared or in a lone pair. Since there are already three N-F bonds in the molecule, there are ten 13 — 3 electron pairs left to be marked on the atoms. It is essential to consider both the ability to have a greater valence and to be the most electropositive element.
Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble.
Additionally, nitrogen has a lower electronegativity value 2. Copy link. In the Lewis structure of the NF 3 molecule, a central nitrogen atom is surrounded by three fluorine atoms. Once the Lewis structure is drawn, the shape of the NF 3 molecule can be determined. There are no charges on nitrogen atom and fluorine atoms. Aluminum oxide Al2O3 , abundant in Earth's crust, is a compound vital in industries, known for hardness, high-temp resistance, and diverse applications In the NF3 Lewis structure, lone pairs are placed on atoms to fulfill the octet rule and achieve electron stability. Nitrogen has a lower electronegativity value 2. Nitrogen trifluoride is a chemical compound with chemical formula NF3. This forms three bonds, using 6 valence electrons. Although NF3 is indispensable in the electronics industry, it is a significant greenhouse gas, and its heat storage capacity is 17, times that of carbon dioxide.

I am sorry, that I interrupt you, but I suggest to go another by.
Now all became clear, many thanks for the information. You have very much helped me.