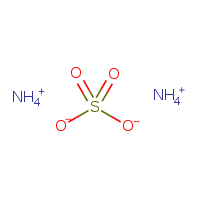
Nh4 2so4
Ammonium Sulfate is non-hazardous to humans. It is also known as Diammonium sulfate or Sulphuric acid diammonium salt.
Ammonium Sulfate has a wide range of uses providing a good source of both sulfur and nitrogen. To report an issue with this product or seller, click here. Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon. It also analyzed reviews to verify trustworthiness. Customers like the quality, ease of use, growth, and speed of the lab chemical. They mention that it dissolves instantly, is water soluble, and produces good growth.
Nh4 2so4
Ammonium sulfate American English and international scientific usage; ammonium sulphate in British English ; NH 4 2 SO 4 , is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. The primary use of ammonium sulfate is as a fertilizer for alkaline soils. In the soil, the ammonium ion is released and forms a small amount of acid, lowering the pH balance of the soil , while contributing essential nitrogen for plant growth. The main disadvantage to the use of ammonium sulfate is its low nitrogen content relative to ammonium nitrate , which elevates transportation costs. It is also used as an agricultural spray adjuvant for water-soluble insecticides , herbicides , and fungicides. There, it functions to bind iron and calcium cations that are present in both well water and plant cells. It is particularly effective as an adjuvant for 2,4-D amine , glyphosate , and glufosinate herbicides. Ammonium sulfate precipitation is a common method for protein purification by precipitation. As the ionic strength of a solution increases, the solubility of proteins in that solution decreases. Ammonium sulfate is extremely soluble in water due to its ionic nature, therefore it can "salt out" proteins by precipitation. The significance of this substance in the purification of compounds stems from its ability to become more so hydrated compared to relatively more nonpolar molecules and so the desirable nonpolar molecules coalesce and precipitate out of the solution in a concentrated form. This method is called salting out and necessitates the use of high salt concentrations that can reliably dissolve in the aqueous mixture. The percentage of the salt used is in comparison to the maximal concentration of the salt in the mixture can dissolve.
Add to Cart. Other cations. See all photos.
.
Ammonium Sulfate is non-hazardous to humans. It is also known as Diammonium sulfate or Sulphuric acid diammonium salt. It has no smell and dissolves in water easily. It does not dissolve in acetone. It appears as a crystalline solid white and has a salty taste. Ammonium sulfate is mainly used as a fertilizer for alkaline soils. The reaction between the sulphuric acid and the ammonium hydroxide produces ammonium sulfate. Sulphuric acid is a strong acid and ammonium hydroxide is a weak base. Therefore, the salt formed by ammonium sulphate is acidic.
Nh4 2so4
Ammonium sulfate American English and international scientific usage; ammonium sulphate in British English ; NH 4 2 SO 4 , is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. The primary use of ammonium sulfate is as a fertilizer for alkaline soils. In the soil, the ammonium ion is released and forms a small amount of acid, lowering the pH balance of the soil , while contributing essential nitrogen for plant growth. The main disadvantage to the use of ammonium sulfate is its low nitrogen content relative to ammonium nitrate , which elevates transportation costs. It is also used as an agricultural spray adjuvant for water-soluble insecticides , herbicides , and fungicides. There, it functions to bind iron and calcium cations that are present in both well water and plant cells. It is particularly effective as an adjuvant for 2,4-D amine , glyphosate , and glufosinate herbicides.
Seminole ranch wma
Insoluble in acetone , alcohol and ether. These items are shipped from and sold by different sellers. I do not compromise when it comes to water quality for them. Reviews with images. Quality Ease of use Growth Speed. Or, substantially less for more with the same results you expect? Not added. Ironically, Green to Clean is a 2 pound Di-ammonium sulfate, more accurately, two pounds of ammonium sulfate per treatment for up to 20, gallons. The Huffington Post. They mention that it dissolves instantly, is water soluble, and produces good growth. Thermodynamics Chemistry. Atoms And Molecules.
Diammonium sulfate. No predicted properties have been calculated for this compound.
Add to Cart. About this item. Ammonium sulfate is made by treating ammonia with sulfuric acid :. Suba December 24, at am. Chemical formula. It is also called oxalate diammonium, or salt diammonium. Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them. It appears as a crystalline solid white and has a salty taste. This method is called salting out and necessitates the use of high salt concentrations that can reliably dissolve in the aqueous mixture. Eligible for Return, Refund or Replacement within 30 days of receipt. OL M. Ammonium sulfate has also been used in flame retardant compositions acting much like diammonium phosphate. It also analyzed reviews to verify trustworthiness. ISBN

0 thoughts on “Nh4 2so4”