N2o lewis dot
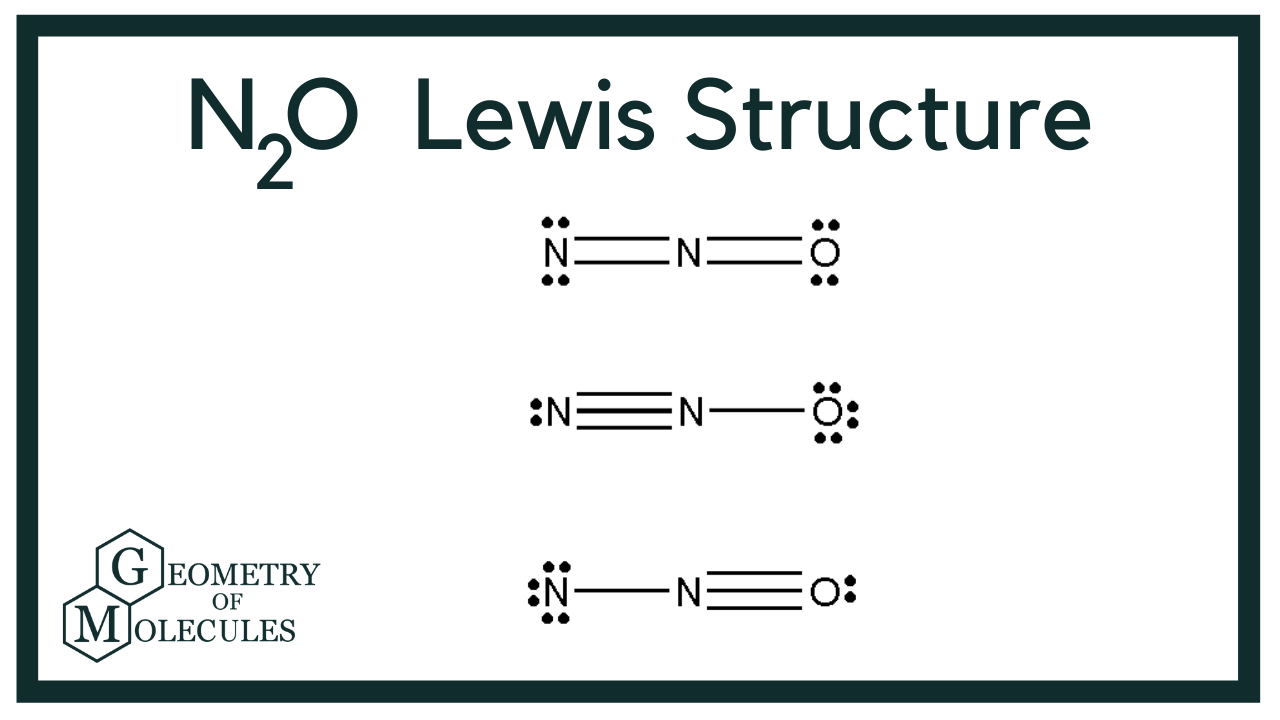
Transcript: Let's do the N2O Lewis structure. N2O has 16 total valence electrons. There's three ways we can draw it, and all of them work pretty well.
Post by » Mon Nov 05, am. Post by chaggard » Mon Nov 05, am. Post by » Tue Nov 06, am. Post by mbaker4E » Tue Nov 06, am. Post by Michael Nirula » Wed Nov 07, am.
N2o lewis dot
I learned this by the counting-electron method, and then assigning formal charges to determine the most likely distribution of valence electrons. We have two nitrogens and one oxygen, which suggests that either we have oxygen in the middle or two nitrogens in a row. Notice how if you had oxygen in the middle , the formal charges of both nitrogens have no way of being distributed well without exceeding 8 electrons for oxygen :. Now we can get two plausible possibilities, which are both linear molecular geometries NOT bent!!! Two electron groups! Truong-Son N. Jan 26, Thus, one of the nitrogens must be in the middle. Related questions How is the Lewis structure of an ion written? Are non-valence electrons represented in a Lewis dot diagram?
The shared pairs are bonding pairs, as represented by the dash or line between the atomic symbols. Each Cl atom now has seven electrons assigned to n2o lewis dot, and the I atom has eight. Strong Titrate-Strong Titrant Curves.
Previously, we discussed how to write Lewis structures for molecules and polyatomic ions. In some cases, however, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. The sum of the formal charges of all atoms in a molecule must be zero; the sum of the formal charges in an ion should equal the charge of the ion.
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. N 2 O is covalent molecule. The central N atom is sp hybridized and terminal N and O are sp, and sp 3 hybridized respectively. Being sp hybridization the geometry of Nitrous Oxide is linear. So, the N-N-O bond angle is 0. The central N makes one covalent bond with N and O. The molecule is neutral but in resonance, its show different canonical form, and some of them are charged. The lone pairs reside over N as well as O. N-N bond makes zero dipole moment but N-O makes resultant dipole moment. So N 2 O is a polar molecule.
N2o lewis dot
The Oxygen atom has 3 lone pairs and the outer nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of N2O. Here, the given molecule is N2O. In order to draw the lewis structure of N2O, first of all you have to find the total number of valence electrons present in the N2O molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Nitrogen is a group 15 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. Here in the N2O molecule, if we compare the nitrogen atom N and oxygen atom O , then the nitrogen is less electronegative than oxygen.
Ts jessyca ketlen
Remember, a Lewis structure is not the molecule, but only a shorthand symbolism that is meant to convey some information about it. For the diatomic hydrogen molecule, H 2 , there are two total valence electrons, so the steps to its Lewis structure are as illustrated below. Crystalline Solids. However, the first arrangement of atoms is preferred because it has the lowest number of atoms with nonzero formal charges Guideline 2. Root Mean Square Speed. The convention for ions is to enclose the structure in brackets, and indicate the net charge at the upper right corner. Hydrohalogenation Reactions. Periodic Trend: Successive Ionization Energies. For acetic acid, the skeletal structure is centered on a chain of atoms bonded together as C C O. If there are additional single electrons present on adjacent atoms, these are also paired in additional bonds. Measuring Radioactivity.
N 2 O nitrous oxide has two nitrogen atoms and one oxygen atom. In the N 2 O Lewis structure, there is a triple bond between two nitrogen atoms, and a single bond between nitrogen and oxygen atom. The left nitrogen atom with a triple bond has one lone pair, and the oxygen atom with a single bond has three lone pairs.
Phase Diagrams. In many cases, the rules that we will develop presently will render a given arrangement less likely, or very implausible. Can Lewis structures predict the shape of a molecule? Solutions: Mass Percent. Henry's Law Calculations. Counting valence electrons yields eight total six from oxygen, one each from the two hydrogens. Chemical Bonds. Chemistry: Structure and Properties, 3rd ed. So the total formal charge for this Nitrogen right here, it's going to be negative 1. In c , we have replaced both bonding pairs with a line or dash to symbolize the covalent bond formed between the atoms by the bonding electron pair. Learning objectives and questions Draw valid Lewis structures for molecules and polyatomic ions Page updated References Tro NJ. Nuclear Binding Energy.

It is well told.
It is interesting. You will not prompt to me, where I can find more information on this question?
I can not recollect.