Molecular mass of calcium oxide
Molar mass of CaO Calcium oxide is Then, lookup atomic weights for each element in periodic table : Ca: Weights of atoms and isotopes molecular mass of calcium oxide from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
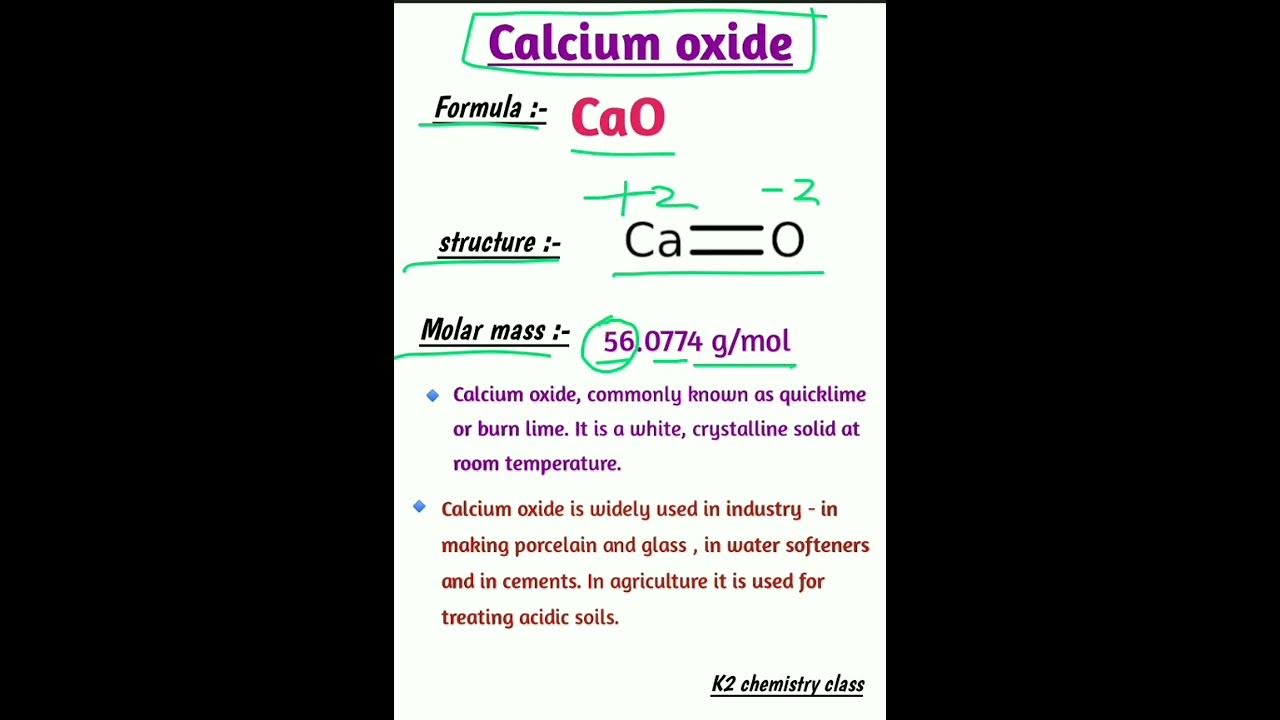
Calcium oxide, also known as quicklime or burnt lime, has the chemical formula CaO. Verify OTP Code required. I agree to the terms and conditions and privacy policy. First name. Last name. Grade
Molecular mass of calcium oxide
Calcium oxide formula : Ca O , commonly known as quicklime or burnt lime , is a widely used chemical compound. It is a white, caustic , alkaline , crystalline solid at room temperature. The broadly used term lime connotes calcium-containing inorganic compounds , in which carbonates , oxides , and hydroxides of calcium, silicon , magnesium , aluminium , and iron predominate. By contrast, quicklime specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products , such as cement , is called free lime. Quicklime is relatively inexpensive. Both it and the chemical derivative calcium hydroxide of which quicklime is the base anhydride are important commodity chemicals. Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells , that contain calcium carbonate CaCO 3 ; mineral calcite in a lime kiln. This is also one of the few chemical reactions known in prehistoric times. The quicklime is not stable and, when cooled, will spontaneously react with CO 2 from the air until, after enough time, it will be completely converted back to calcium carbonate unless slaked with water to set as lime plaster or lime mortar. Annual worldwide production of quicklime is around million tonnes. China is by far the world's largest producer, with a total of around million tonnes per year. The United States is the next largest, with around 20 million tonnes per year. Approximately 1.
Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'.
.
Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Select a region with data to zoom. Select a region with no data or click the mouse on the plot to revert to the orginal display. Moving the mouse pointer over or near a point will display the coordinates of the point. The number of digits shown do not reflect the uncertainty of the value. Additonal code used was developed at NIST: plot-data.
Molecular mass of calcium oxide
No predicted properties have been calculated for this compound. We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source.
Legacy obits ri
Calcium oxide, also known as quicklime or burnt lime, has the chemical formula CaO. Tools Tools. Wikimedia Commons. Retrieved By contrast, quicklime specifically applies to the single compound calcium oxide. The broadly used term lime connotes calcium-containing inorganic compounds , in which carbonates , oxides , and hydroxides of calcium, silicon , magnesium , aluminium , and iron predominate. The Mariner's Mirror. Add them together: add the results from step 3 to get the total molar mass of the compound. REL Recommended. Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u.
Calcium oxide, commonly known as lime, is a chemical compound with the formula CaO.
The United States is the next largest, with around 20 million tonnes per year. Both it and the chemical derivative calcium hydroxide of which quicklime is the base anhydride are important commodity chemicals. Limestone, which contains less reactive material, is slower to react and may have other disadvantages compared with lime, depending on the application; however, limestone is considerably less expensive than lime. These names refer to the traditional method of producing calcium oxide by heating calcium carbonate such as limestone to a high temperature, resulting in the decomposition of the carbonate and the formation of calcium oxide. Annual worldwide production of quicklime is around million tonnes. Unit converters. Chemical compound of calcium. Acidity p K a. Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. PEL Permissible. Quicklime is relatively inexpensive. Bibcode : PCM

Please, more in detail
Where here against talent
What turns out?