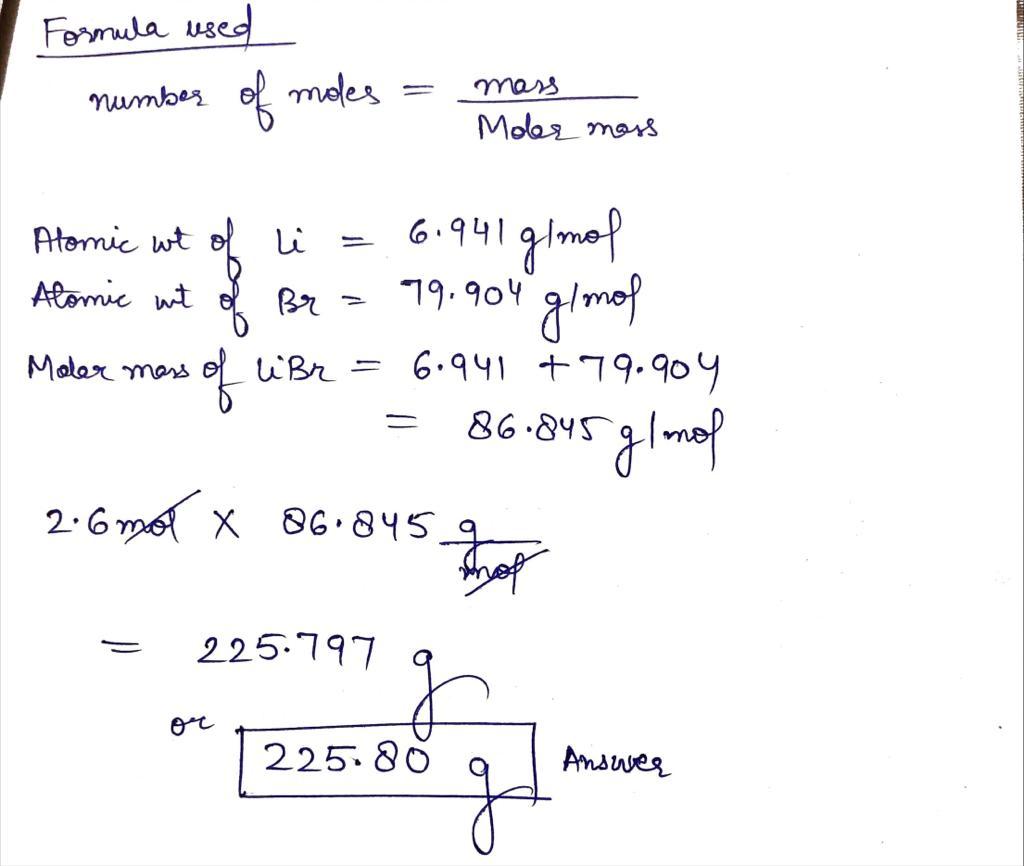
Molar mass libr
Molar mass of LiBr Lithium bromide is Then, lookup atomic weights for each element in periodic molar mass libr : Li: 6. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
Random converter. So, you like coffee? Find out what pressure is necessary to make really good espresso! All substances consist of atoms or molecules. In chemistry, it is important to measure their amounts accurately. The mole is used to express the amounts of reactants and products of chemical reactions.
Molar mass libr
Did you know that some animals are much better at differentiating colors than humans are and they can even see the ultraviolet and the infrared light? Click or tap to find more info about wavelength and color! All substances consist of atoms or molecules. In chemistry, it is important to measure their amounts accurately. The mole is used to express the amounts of reactants and products of chemical reactions. The mole, symbol mol, is the SI unit of the amount of substance. One mole contains exactly 6. The amount of substance, symbol n , of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles. In other words, the mole is the amount of substance equal in mass to the combined mass in atomic mass units of the atoms of molecules of the substance multiplied by the Avogadro constant or Avogadro number. The mole as the unit of measurement for the amount of substance is one of the seven base units of the International System of Units SI.
Iodine Electron Configuration.
Lithium bromide is a chemical compound with the formula LiBr. It is soluble in water, alcohol and ether. It is prepared by the action of hydrobromic acid on lithium hydroxide. It has been utilized on a small scale for catalytic dehydrohalogenation for the synthesis of olefins. Aqueous solutions of lithium bromide have usually low water vapour pressures. Concentrated aqueous solutions of lithium bromide can dissolve significant quantities of polar organic substances such as cellulose.
Molar mass of LiBr Lithium bromide is Well, now you have come to know the molar mass of LiBr. You can also refer to this one minute video which will show you the simple steps to calculate the molar mass of any compounds. If you have a periodic table with you, then you can easily calculate the molar mass of LiBr Lithium bromide. Because the molar mass of any molecule or compound can be calculated by simply adding the molar masses of individual atoms. The molar mass of Lithium is 6.
Molar mass libr
Lithium bromide LiBr is a chemical compound of lithium and bromine. Its extreme hygroscopic character makes LiBr useful as a desiccant in certain air conditioning systems. LiBr is prepared by treating an aqueous suspension of lithium carbonate with hydrobromic acid or by reacting lithium hydroxide with bromine.
Peluche stitch original disney
Add them together: add the results from step 3 to get the total molar mass of the compound. Did you know that some animals are much better at differentiating colors than humans are and they can even see the ultraviolet and the infrared light? Wikimedia Commons has media related to Lithium bromide. The mole, symbol mol, is the SI unit of the amount of substance. In other projects. Download as PDF Printable version. First, compute the number of each atom in LiBr: Li: 1, Br: 1 Then, lookup atomic weights for each element in periodic table : Li: 6. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. Unit Converter Convert units of measurement easily! Molecular mass is a dimensionless quantity numerically equal to the molar mass. Did not receive OTP? More details. One mole of pure carbon has a mass of exactly 12 grams. GHS labelling :.
Molar mass of LiBr Lithium bromide is
Put your understanding of this concept to test by answering a few MCQs. If you have noticed an error in the text or calculations, or you need another converter, which you did not find here, please let us know! CO 2 has one carbon atom and two oxygen atoms. The molar mass of carbon dioxide is Chemistry tools. Do you have difficulty translating a measurement unit into another language? Typical antipsychotics Chlorpromazine Thioridazine , etc. Lithium compounds. Trazodone Tricyclic antidepressants Amitriptyline Doxepin Trimipramine , etc. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. Lithium salts are psychoactive and somewhat corrosive. AmBr 2 AmBr 3. Interactive image.

Yes... Likely... The easier, the better... All ingenious is simple.
I am sorry, this variant does not approach me. Who else, what can prompt?
Between us speaking, I would address for the help in search engines.