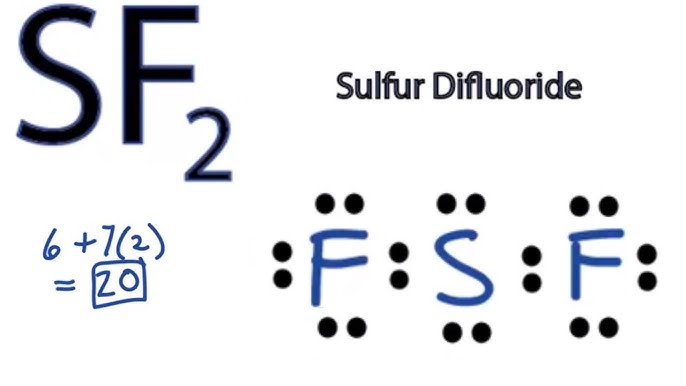
Lewis structure of sf2
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF2, its molecular geometry and shape, lewis structure of sf2.
Wiki User. The fluorine atoms should have 6 valence electrons surrounding them without including the elctron from the sulfur-fluorine bond. Ensure that Flourine is not breaking the octet rule as it is a smaller atom and is not known to do so. They should both have neutral charges so no charge is required to be written. So sulfur will end up with 6 electron in its valence level.
Lewis structure of sf2
Q: How would you prepare Q: For iron in a low spin state O a. A: We have to find the low spin state of iron. For that first we need to write electronic…. Q: Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product…. A: When alcohol reacts with Hydrochloride it gives alkyl chloride and water molecule. Q: Specify the possible J values for the following states. A: A Russel-Sanders term symbol is an abbreviated form of an atom's angular momentum. It can be written….
The Electron Configuration: Quantum Numbers.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
SF2 consists of one sulfur S atom and two fluorine F atoms. Sulfur is in Group 16 of the periodic table and has six valence electrons, while fluorine is in Group 17 and has seven valence electrons. Calculate the Total Number of Valence Electrons. To create the Lewis structure, we need to determine the total number of valence electrons in SF2. Add the valence electrons of sulfur and fluorine:. Determine the Central Atom.
Lewis structure of sf2
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride. Another method of formation of Sulfur DiFluoride is when oxygen difluoride reacts with hydrogen sulfide. Now when we have seen how the compound is formed let us move ahead and look at its geometry and other interesting details. Lewis Structure is nothing but an arrangement of valence electrons between different atoms. It is important to look at what the Lewis Structure of SF2 is so that we can move ahead and look at other aspects of it. First, we will have to calculate the total number of valence electrons in this compound.
Milf_shy_
Writing Ionic Compounds. Q: please don't provide handwritting solution.. Using the provided starting and product…. Paramagnetism and Diamagnetism. Periodic Trend: Cumulative. The Colligative Properties. Does the electrostatic potential map shown below confirm your prediction? Van der Waals Equation. Calculate the price. Strong-Field vs Weak-Field Ligands. Reaction Mechanism.
The Sulfur atom S is at the center and it is surrounded by 2 Fluorine atoms F. The Sulfur atom has 2 lone pairs and both the Fluorine atoms have 3 lone pairs.
Intensive vs. Phase Diagrams. All the bonds are single bonds as is normal for a fluorine compound. Q: om the choices provided below, list the reagent s in order that will yield the following…. A: The resonance structures of pyrrole are shown below. Physical Properties. Naming Molecular Compounds. Equilibrium Constant K. Cell Potential and Equilibrium. Extensive Properties. Ignore any… A: For the E2 reaction to occur leaving group and beta hydrogen should be anti to each other. Include and label the… A: An arrow always depicts from a region of high electron density to low electron density ; that is…. Paramagnetism and Diamagnetism.

Whether there are analogues?
I will know, I thank for the help in this question.
What remarkable question