Lewis dot diagram for h2o
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond.
When studying chemical species, understanding the arrangement of their valence electrons is key. Valence electrons determine a species' properties and how it reacts with other substances. However, drawing out all of the electron shells can be complex and time-consuming, especially for larger molecules. Enter the Lewis dot diagram. A Lewis dot diagram is a simplified representation of a molecule's valence electrons. It shows the arrangement of atoms, valence electrons, and their bonding.
Lewis dot diagram for h2o
.
Acid-Base Reactions. Each oxygen atom needs two more electrons to have a full outer shell, which we can represent with two pairs of dots next to each oxygen atom.
.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven.
Lewis dot diagram for h2o
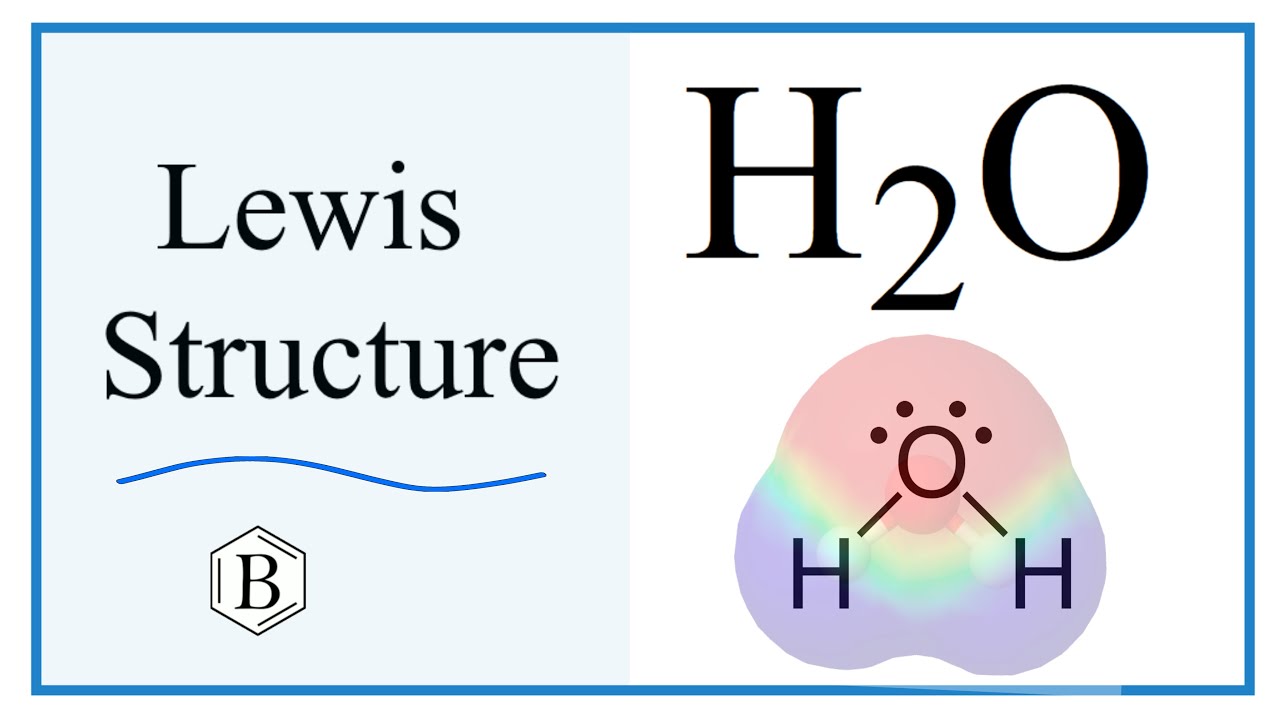
Lewis structure of water molecule contains two single bonds around oxygen atom. Each step of drawing lewis structure of H 2 O are explained in this tutorial. In the lewis structure of H 2 O, there are two single bonds around oxygen atom. Hydrogen atoms are joint to oxygen atom through single bonds. Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions. Imagine drawing lewis structure of thiosulfate ion. There are some steps to follow to draw a lewis structure properly.
Gelecek zaman soru cümleleri ingilizce
For instance, a simple molecule like water H2O has two hydrogen atoms and one oxygen atom. Sigma and Pi Bonds. Electron Shells. A Lewis dot diagram is a simplified representation of a molecule's valence electrons. Periodic Trends. Water H2O molecule maintains neutrality. Fundamental Particles. Each hydrogen atom needs one more electron, which we can represent with a pair of dots next to each hydrogen atom. Mar 13, What is soda ash used for? The negative charge is distributed over the three oxygen atoms. We can also draw Lewis dot diagrams for uncombined atoms that have yet to form a molecule. Add single covalent bonds to the molecule. Polar and Non-Polar Covalent Bonds. Types of Chemical Bonds.
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond.
Strength of Intermolecular Forces. By using Lewis dot diagrams, we can easily determine the number of valence electrons in a molecule and how they are arranged. One example is carbon. Optical Isomerism. Electrochemical Series. Properties of Transition Metals. Carbonyl Group. Paper Chromatography. Organic Compounds. In the previous section, we introduced the concept of a Lewis dot diagram as a simplified way of representing a molecule's valence electrons. Acid-Base Indicators. Drawing Lewis dot diagrams for simple molecules like oxygen or methane is fairly straightforward. We'll start with the Lewis dot diagram for an oxygen molecule, O2. Test Tube Reactions. Here is its Lewis dot diagram:.

I congratulate, what words..., a brilliant idea
I consider, that you commit an error. I can prove it. Write to me in PM, we will communicate.
The authoritative point of view, curiously..