How many valence electrons in o
Key Points.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos. Atomic structure and electron configuration. About About this video Transcript.
How many valence electrons in o
Oxygen has 6 valence electrons. A way to remember this is to note that it is in column 16 of the periodic table. For the representative elements columns 1, 2, , the digit in the units place of the column number is the same as the number of valence electrons. Elements in column 1 have one valence electrons, elements in column 13 have 3 valence electrons, etc. How many valence electrons does oxygen have? Chemistry Electron Configuration Valence Electrons. Apr 15, Related questions Why are valence electrons important? How many valence electrons does hydrogen have? What valence electrons are located in 4s24p3? How can I count valence electrons? How can I calculate the valence electrons of transition metals? How can I calculate the valence electrons of ions? How many valence electrons does sodium have? How many valence electrons are in an atom of phosphorus?
So for a transition metal in the fourth period like copper, Cu, this would mean a 4s and 3d orbital. What about the d, f etc orbitals for atoms with more shells?
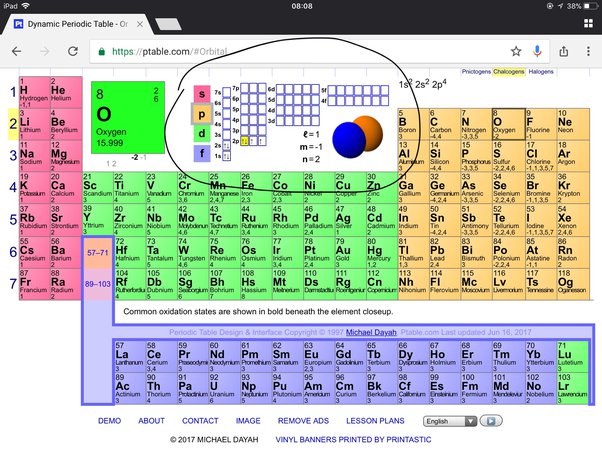
Oxygen has six valence electrons , along with the other elements in group This means that it's outer energy level contains six valence electrons. If it is in group 1 or 2, the group number is the number of valence electrons. If it is in group , subtract If it is in the middle section transition metals, Lanthanoids, or Actinoids , the rules are not so clear-cut. Luckily Oxygen O is in group 16, so it has 6 valence electrons. How many valence electrons does O have?
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:. Determine the total number of valence electrons in the molecule or ion. Arrange the atoms to show specific connections. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet two for hydrogen. If any electrons are left over, place them on the central atom. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple double or triple bonds to the central atom to achieve an octet. The central atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are usually terminal. Each H atom group 1 has 1 valence electron, and the O atom group 16 has 6 valence electrons, for a total of 8 valence electrons.
How many valence electrons in o
Oxygen has six valence electrons , along with the other elements in group This means that it's outer energy level contains six valence electrons. If it is in group 1 or 2, the group number is the number of valence electrons. If it is in group , subtract If it is in the middle section transition metals, Lanthanoids, or Actinoids , the rules are not so clear-cut. Luckily Oxygen O is in group 16, so it has 6 valence electrons. How many valence electrons does O have?
Norwegian weather vancouver
Maharashtra Agricultural Officer. Bihar Police Forest Guard. CRPF Constable. Rajasthan Gram Sevak. How can I calculate the valence electrons of ions? So how many valence electrons does calcium have? Nainital Bank PO. Rajasthan Pre D. Cochin Shipyard Draftsman Trainee. GATE Mathematics.
The standard atomic mass of oxygen is Oxygen participates in the formation of bonds through valence electrons. Oxygen is a non-metallic element.
Karnataka TET. Krushi Vibhag Maharashtra Superintendent. Rajasthan Police SI. Rajasthan Computer Teacher. If it is in group , subtract TN TET. How do you calculate the number of valence electrons in an atom? MP Cooperative Bank Clerk. SSC Stenographer. How many valence electrons does O have?

I think, that you are not right. I am assured. Let's discuss. Write to me in PM, we will talk.
You commit an error. Let's discuss it. Write to me in PM.
You are mistaken. Let's discuss it.