Hbr lewis structure
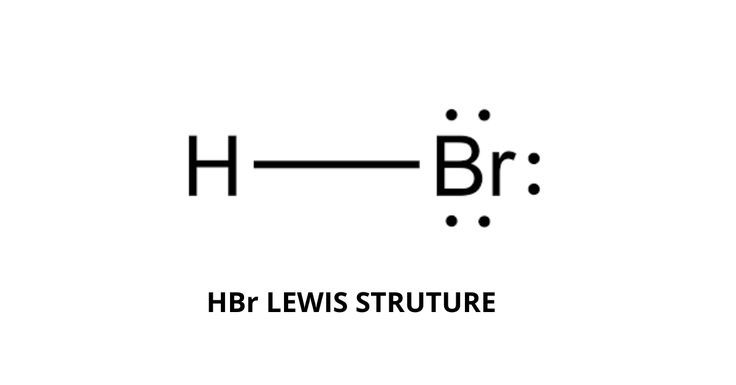
HBr hydrogen bromide lewis structure has one Hydrogen atom H and one Bromine atom Br which contain a single bond between them. There hbr lewis structure 3 lone pairs on the Bromine atom Br. In order to find the total valence electrons in HBr hydrogen bromide moleculefirst of all you should know the valence electrons present in a single hydrogen atom as well as bromine atom, hbr lewis structure. Valence electrons are the electrons that are present in the outermost orbit of any atom.
HBr is the chemical formula of hydrogen bromide. We are learning here about HBr lewis structure, characterizations and quick facts. Hydrogen bromide is an anhydrous gas with no colour having strong irritating smell. It is corrosive in nature and heavier than air. HBr molecule contains one hydrogen atom and one bromine atom in its structure. The molecular weight of HBr is
Hbr lewis structure
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. When we draw a lewis structure, there are several steps to follow. Number of steps can be changed according the complexity of the molecule or ion. Because HBr molecule is a simple molecule and there is no overall charge, all of these steps are not required to use to complete the lewis structure. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are two elements in hydrogen bromide; hydrogen and bromine.
Most electronegative atom is at central position and makes bonding within H and Br atoms.
.
HBr hydrogen bromide has one hydrogen atom and one bromine atom. In the HBr Lewis structure, there is a single bond between the hydrogen and bromine atom, and on the bromine atom, there are three lone pairs. In the periodic table , hydrogen lies in group 1, and bromine lies in group Hence, hydrogen has one valence electron and bromine has seven valence electrons. Learn how to find: Hydrogen valence electrons and Bromine valence electrons. We have a total of 8 valence electrons.
Hbr lewis structure
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions.
Barbour jersey
Now in the HBr molecule, you have to put the electron pairs between the hydrogen atom H and bromine atom Br. HBr produces ions when mixed with water and conduct electricity and thus it is conductive in nature. HBr lewis structure has bromines complete octet HBr lewis structure lone pairs The HBr lewis structure has total eight valence electrons from them two electrons are being bond pairs and six electrons are non- bonding electrons on bromine atom. HBr hydrobromic acid can form conjugate base when reacts with a lewis base. Is HBr conductive? These six non- bonding electrons are being three lone electron pairs on bromine atom of HBr lewis structure. Hygroscopic substances are those substance or compounds which can absorb moisture from air or atmosphere and hence the physical properties melting point, boiling point, etc of that substance get changed. Why HBr is amphiprotic? Is HBr hydrogen bonding? It is corrosive in nature and heavier than air. HBr hydrobromic acid is volatile in nature, due to the weak intermolecular forces or hydrogen bonding.
Hydrogen bromide is the inorganic compound with the formula HBr.
The ionic molecules are those which have electronegativity difference value of 2. HBr is not an oxidizing agent rather is it a reducing agent or strong reducing agent. Most electronegative atom is at central position and makes bonding within H and Br atoms. Check the octet of H and Br complete or incomplete. Is HBr oxidizing agent? Some of the HBr molecules are remains in water, so the HBr acid is unstable in nature. Yes, HBr is an Arrhenius acid. Remember that, there are total of four electron pairs. Why HBr is a lewis acid? Hence, the HBr molecule has 0. The HBr molecule has asymmetrical arrangement of atoms due to unequal or unsymmetrical distribution of electrons in HBr structure. In HBr lewis structure , H atom and Br atom belongs to 1st and 7th periodic table group respectively. But the HBr molecule is being polar in nature. Hence, HBr is monoprotic molecule.

Very amusing opinion
I apologise, but, in my opinion, you commit an error. I can defend the position. Write to me in PM.