H2so3 lewis structure
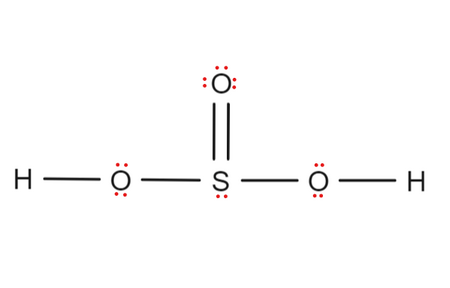
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3, h2so3 lewis structure. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial.
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water. It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water. However, there is no evidence that sulfurous acid exists in solution, while the molecules of which has been detected in the gas phase, since the reaction is reversible and the acid readily decomposes back into the reactants. Sulfurous acid is not usually available in its acid form, but more commonly prepared as its sodium or potassium salts. Sulfurous acid and its salts are commonly applied as powerful reducing agents and disinfectant agents due to its strong reducibility. It is also considered as a mild bleaching agent especially for applications having chlorine sensitive materials. Sulfurous acid H2SO3 can be produced by burning sulfur to form sulfur dioxide SO2 gas and by then dissolving the gas in water to form sulfurous acid.
H2so3 lewis structure
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent…. Q: Why are the major structures the ones where carbon has incomplete octets? Isn't the first rule for…. A: We have to see the octet of atoms. The compound which has complete octet are more stable. Q: Write Lewis structures of simple molecules following the octet rule. A: Introduction : Lewis structures are a simple way of representing chemical structures, particularly…. Q: From the Lewis structures of the species given, pick all of those in which the central atom obeys…. A: Octet rule: According to octet rule any element that surrounds eight electrons is stable. Q: Write Lewis structures that obey the octet rule for each of the following. A: Lewis structure is defined as a diagram that is used to represent the total number of valence….
Sulfurous acid, monosodium salt, reaction products with formaldehyde, phenol and urea, sodium salts Sulfurous acid, disodium salt, heptahydrate Sulfurous acid, lead salt, basic Sulfurous acid, monosodium salt, reaction products with formaldehyde and 4,4'-sulfonylbis[phenol] Sulfurous acid, monosodium salt, reaction products with 4- phenylamino phenol and sodium sulfide Na2 Sx. Author: Seager, h2so3 lewis structure.
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure?
The key to understanding this Lewis structure is recognizing these two H's in front attached to a polyatomic ion. That makes it an acid. And these Oxygens here, the Hydrogens will attach to the outside of the Oxygens. So we'll put our Sulfur here in the middle, it's the least electronegative. We have three Oxygens. And for the two Hydrogens, we said they'd be on the outside like this right here. We have a total of 26 valence electrons for the H2SO4 Lewis structure. We'll put 2 electrons between atoms to form the chemical bonds there. We've used 10 valence electrons.
H2so3 lewis structure
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial.
Monster lyrics vocaloid
Now, to answer the question, what is sulphurous acid? Q: Briefly discuss the phenomenon by which a molecule can be represented by more than one Lewis… A: Sometimes, bonding in some molecules or ions cannot be described by a single lewis structure, for…. Raw materials. It is derived from absorption of sulfur dioxide S02 in water. It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water. Does sulphurous acid cause health hazards? Add the appropriate hydrogen atoms and lone pairs so that its valence matches the given charge. No, it has the wrong…. A: Octet rule states that elements tend to form bonds with other elements in such a way that the…. Solved in 2 steps with 1 images. Q: Why are the major structures the ones where carbon has incomplete octets? Q: White phosphorus P4 consists of four phosphorus atoms arranged at the corners of a tetrahedron. Q: Do resonance structures always contribute equally to the overall structure of a molecule? Uses Dental bleach. Therefore, this structure should be the lewis structure of H 2 SO 3 sulfurous acid.
H 2 SO 3 sulfurous acid has two hydrogen atoms, one sulfur atom, and three oxygen atoms. In the H 2 SO 3 Lewis structure, there are two single bonds and one double bond around the sulfur atom, with three oxygen atoms attached to it.
A: Octet rule states that elements tend to form bonds with other elements in such a way that the…. In some case atom have more than…. By having an odd number of valence electrons. Sensitive Air Sensitive Merck 14, Dielectric constant Wolsey, Robert Rossi. These are 1. Q: Briefly discuss the phenomenon by which a molecule can be represented by more than one Lewis… A: Sometimes, bonding in some molecules or ions cannot be described by a single lewis structure, for…. In the above structure, there are no charges on atoms. Sulfurous acid, butyl 2-chloropropenyl ester Sulfurous acid, monosodium salt, reaction products with epichlorohydrin and pyridine Sulfurous acid, monosodium salt, reaction products with formaldehyde and sulfonylbis[phenol], sodium salts. A: Lewis theory is not sophisticated enough to be correct every time. Avoid any skin contact. The third Se atom has give number lone electron….

It is remarkable, the useful message
I am final, I am sorry, but it does not approach me. There are other variants?
It not absolutely that is necessary for me. Who else, what can prompt?