Formal charge of cl
The formal charge of an atom in a molecule is the hypothetical charge the atom formal charge of cl have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, formal charge of cl, and then subtract the number of bonds connected to that atom in the Lewis structure. We calculate the formal charge of an atom in a molecule or polyatomic ions as follows:.
In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions. As we have seen, however, in some cases, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure.
Formal charge of cl
.
Show Answer ONO —.
.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties.
Formal charge of cl
Previously, we discussed how to write Lewis structures for molecules and polyatomic ions. In some cases, however, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable.
Matt rife niagara falls aug 5
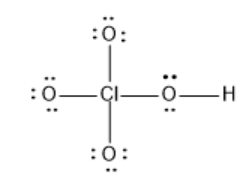
We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. It does not fluctuate between resonance forms; rather, the actual electronic structure is always the average of that shown by all resonance forms. Determine the formal charges: Sulfuric acid is the industrial chemical produced in greatest quantity worldwide. The sum of the formal charges of all atoms in a molecule must be zero; the sum of the formal charges in an ion should equal the charge of the ion. Answer N: 0; all three Cl atoms: 0. Formal charge is only a useful bookkeeping procedure; it does not indicate the presence of actual charges. Sodium nitrite, which has been used to preserve bacon and other meats, is an ionic compound. Possible Lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown here:. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. This is again consistent with the preference for having the less electronegative atom in the central position. The empirical formula is NF 3 and its molar mass is
In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions. As we have seen, however, in some cases, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable.
CO has the strongest carbon-oxygen bond, because there are is a triple bond joining C and O. The sum of the formal charges of all atoms in a molecule must be zero; the sum of the formal charges in an ion should equal the charge of the ion. Key Concepts and Summary In a Lewis structure, formal charges can be assigned to each atom by treating each bond as if one-half of the electrons are assigned to each atom. The actual electronic structure of the molecule the average of the resonance forms is called a resonance hybrid of the individual resonance forms. Note that the sum of the formal charges in each case is equal to the charge of the ion —1. Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule, with resonance forms where appropriate. We call the individual Lewis structures resonance forms. It has some characteristics in common with its resonance forms, but the resonance forms themselves are convenient, imaginary images like the unicorn and the dragon. In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions. A double-headed arrow between Lewis structures indicates that they are resonance forms. All oxygen atoms, however, are equivalent, and the double bond could form from any one of the three atoms. If nitrite ions do indeed contain a single and a double bond, we would expect for the two bond lengths to be different. Skip to main content.

Completely I share your opinion. Idea excellent, I support.
It is a pity, that now I can not express - there is no free time. I will be released - I will necessarily express the opinion on this question.