Equivalent resonance structures
Revolutionized is reader-supported. When you buy through links on our site, we may earn an affiliate commision. Learn more here. Chemists must know about equivalent resonance structures in their work.
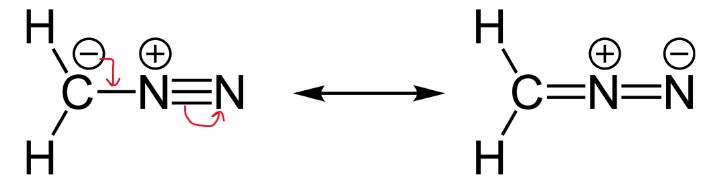
Lewis formulas are misleading in the sense that atoms and electrons are shown as being static. By being essentially two-dimensional representations they also fail to give an accurate idea of the three-dimensional features of the molecule, such as actual bond angles and topography of the molecular frame. Furthermore, a given compound can have several valid Lewis formulas. For example CH 3 CNO can be represented by at least three different but valid Lewis structures called resonance forms, or resonance structures , shown below. However, a stable compound such as the above does not exist in multiple states represented by structures I, or II, or III.
Equivalent resonance structures
In cases in which more than one reasonable plausible Lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors. Resonance structures can be either equivalent or non-equivalent. However, they are not really identical or the same , they are just equivalent. Each structure is called a resonance structure, and they can be connected by the double-headed resonance arrow. There are three equivalent resonance structures for CO 3 2- , and the actual structure of CO 3 2- is a hybrid of the three resonance contributors. Since the resonance structures are equivalent, they are all in the same level of energy and have the same stability, so they make the same contributions to the actual structure of CO This is supported by experimental evidence showing that all the carbon-oxygen bonds in CO are the same bond length, which is longer than a regular double bond but shorter than a single bond. As a result of the resonance structures, the two negative charges in CO are not localized on any oxygen atoms, but are spread evenly among all three oxygen atoms, and this is called charge delocalization. Because of charge delocalization, each oxygen atom has two-thirds of a full negative charge. Charge delocalization helps stabilize the whole species. The stability a species gains from having charge delocalization through resonance contributors is called the resonance stabilization effect. The greater the number of resonance contributors, the greater the resonance stabilization effect and the more stable the species is.
The basic bonding pattern, or connectivityis the same in all structures, but some electrons have changed locations.
A resonance form is another way of drawing a Lewis dot structure for a given compound. Equivalent Lewis structures are called resonance forms. They are used when there is more than one way to place double bonds and lone pairs on atoms. Resonance structures arise when there are more than one way to draw a Lewis dot diagram that satisfies the octet rule. Remember the octet rule is where the atom gains, loses, or shares electrons so that the outer electron shell has eight electrons. We draw them when one structure does not accurately show the real structure.
Resonance structures are a set of two or more Lewis Structures that collectively describe the electronic bonding of a single polyatomic species including fractional bonds and fractional charges. Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integral number of covalent bonds. Sometimes, even when formal charges are considered, the bonding in some molecules or ions cannot be described by a single Lewis structure. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula. A molecule or ion with such delocalized electrons is represented by several contributing structures also called resonance structures or canonical forms. Each O atom has 6 valence electrons, for a total of 18 valence electrons. Assigning one bonding pair of electrons to each oxygen—oxygen bond gives. If we place three lone pairs of electrons on each terminal oxygen, we obtain. At this point, both terminal oxygen atoms have octets of electrons.
Equivalent resonance structures
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Resonance structures. About About this video Transcript. Introduction to resonance structures, when they are used, and how they are drawn. Created by Jay.
Keralasax
These rules will be examined in detail in a later paper. People study chemical reactions while looking for new commercial drugs. Introduction There are some basic principle on the resonance theory. In the resonance forms shown above the atoms remain in one place. In cases in which more than one reasonable plausible Lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors. Atoms that are missing one or more electrons will have a positive charge. Draw all of the resonance structures for azide anion, N 3 — , and indicate the most stable o ne. North Carolina State University offers a free and interactive Lewis structure builder. A resonance form is another way of drawing a Lewis dot structure for a given compound. Maillard reactions are particularly relevant to chemists working for food companies. Figure 1. Recent Articles. Finally, another factor that comes into play when determining the relative energies of resonance structures is the relative electronegativities of atoms that bear charges.
Looking at the structure of formaldehyde we can see that there is a double bond between the central carbon atom and the oxygen atom giving a CO bond order of two. The carbon is singly bonded to each hydrogen atom, which would give each CH bond orders of one. Bond order is an index of bond strength: the higher the bond order, the stronger the bond.
The tail of the arrow begins at the electron source and the head points to where the electron will be. By convention, we use double-headed arrows to indicate that several resonance structures contribute to the same hybrid. Make sure the arrows are clear including the single and half headed arrow. Electrons do not move toward a sp 3 hybridized carbon because there is no room for the electrons. These important details can ensure success in drawing any Resonance structure. Charge separation is an important one. They want that to change, and this is a step in the right direction. The factors that make up valid Lewis formulas are as follows. The delocalized charges can also be represented by the calculated electrostatic potential map of the electron density in the CO 3 2- anion. First resonance structures are not real, they just show possible structures for a compound.

0 thoughts on “Equivalent resonance structures”