Density of hcl solutions
Rather, they are aqueous solutions of these substances in the form of the hydronium ion and the conjugate base. It is important to distinguish between acid solutions and the formula units of the acids which were dissolved, density of hcl solutions. Examine the table below.
Hydrochloric acid is an inorganic acid with the chemical formula HCl. It is classified as a strong acid and can attack the skin. It is a colorless liquid which has a distinctive, pungent smell. Besides its most common use in refining metals, hydrochloric acid is an important chemical used in the production of organic compounds, such as polyvinyl chloride for plastic. We use cookies on our website. Some of them are necessary e.
Density of hcl solutions
Hydrochloric acid , also known as muriatic acid or spirits of salt , is an aqueous solution of hydrogen chloride HCl. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Because it was produced from rock salt according to the methods of Johann Rudolph Glauber , hydrochloric acid was historically called by European alchemists spirits of salt or acidum salis salt acid. Gaseous HCl was called marine acid air. The name muriatic acid has the same origin muriatic means "pertaining to brine or salt", hence muriate means hydrochloride , and this name is still sometimes used. In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi c. After adding an equal weight of good crystallised Sal-ammoniac, dissolve by moisture, and distil the mixture. There will distil over a strong water, which will cleave stone sakhr instantly. However, it appears that in most of his experiments al-Razi disregarded the gaseous products, concentrating instead on the color changes that could be effected in the residue. Multhauf , hydrogen chloride was produced many times without clear recognition that, by dissolving it in water, hydrochloric acid may be produced. Drawing on al-Razi's experiments, the De aluminibus et salibus "On Alums and Salts" , an eleventh- or twelfth-century Arabic text falsely attributed to al-Razi and translated into Latin by Gerard of Cremona — , described the heating of metals with various salts, which in the case of mercury resulted in the production of mercury II chloride corrosive sublimate.
Privacy Policy Legal Notice. For the gas, see hydrogen chloride. Because it was produced from rock salt according to the methods of Johann Rudolph Glauberhydrochloric acid was historically called by European alchemists spirits of salt or acidum salis salt acid.
Steffen's Chemistry Pages. Return to Density tables. Average rating 4. Vote count: No votes so far!
Hydrochloric acid is an inorganic acid with the chemical formula HCl. It is classified as a strong acid and can attack the skin. It is a colorless liquid which has a distinctive, pungent smell. Besides its most common use in refining metals, hydrochloric acid is an important chemical used in the production of organic compounds, such as polyvinyl chloride for plastic. We use cookies on our website.
Density of hcl solutions
Density and percent composition are important properties in chemistry. Each have basic components as well as broad applications. Components of density are: mass and volume, both of which can be more confusing than at first glance.
Tresso
Rather, they are aqueous solutions of these substances in the form of the hydronium ion and the conjugate base. Hydrochloric acid is the preferred acid in titration for determining the amount of bases. McGraw-Hill Book Company. The knowledge of mineral acids such as hydrochloric acid would be of key importance to seventeenth-century chemists like Daniel Sennert — and Robert Boyle — , who used their capability to rapidly dissolve metals in their demonstrations of the composite nature of bodies. I accept all cookies including US-providers Back. Vapour pressure values are taken from the International Critical Tables and refer to the total vapour pressure of the solution. PbCl 2 PbCl 4. Calculations for synthetic reactions where a strong mineral acid is used. Bibcode : JPCA.. The molecular weight of HCl is Typical products include aspartame , fructose , citric acid , lysine , hydrolyzed vegetable protein as food enhancer, and in gelatin production. The first clear instance of the preparation of hydrochloric acid appears in the writings of Della Porta, and , Libavius , pseudo-Basil , van Helmont and Glauber To convert volume to mass, we need the density of the solution.
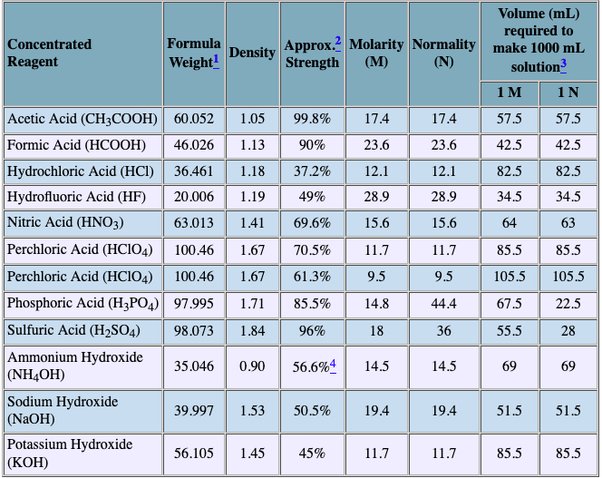
Last term, we introduced molarity , a very useful measurement unit for evaluating the concentration of solutions. However, molarity is only one measure of concentration.
A new industrial process developed by Nicolas Leblanc of Issoudun, France enabled cheap large-scale production of sodium carbonate soda ash. Besides its most common use in refining metals, hydrochloric acid is an important chemical used in the production of organic compounds, such as polyvinyl chloride for plastic. If The resulting hydrogen chloride gas is absorbed in deionized water , resulting in chemically pure hydrochloric acid. It is also inexpensive. Calculations for synthetic reactions where a strong mineral acid is used. Toggle search form Search for:. Analysis 0 We collect and combine data on our visitors and their behavior on our website. Other names Muriatic acid [1] Spirits of salt [2] Hydronium chloride Chlorhydric acid. N verify what is Y N? New York: Prentice Hall. Hydrochloric acid can be used to regulate the acidity pH of solutions. Bibcode : JPCA.. ICl ICl 3. JSTOR

Quite right! Idea excellent, I support.