C2h2 lewis structure
Some substance identifiers may have been claimed confidential, or may not have been provided, and therefore not be displayed.
This introductory chapter describes the simple ideas of atoms and molecules, types of chemical formula and their molecular weight for students who have not studied chemistry before. Chemical equations and balanced chemical equations are introduced through the reactions used in an introductory practical laboratory course. The concepts of molarity and molar solutions are introduced through solving volumetric problems, to enable the student to start a laboratory course in practical Inorganic Chemistry. Chemistry is the science and study of the material world. It is generally accepted that there are three states of matter, solid, liquid and gaseous, and the chemicals that make up the materials of the world involve the chemical elements or molecules.
C2h2 lewis structure
Problemy z witryną? Pytania dotyczące licencji? Skontaktuj się z menedżerem ds. Metoda płatności Dodaj metodę płatności. Dodaj płatność Zmień metodę. Modele 3D. Przejdź do PixelSquid. Gotowe do projektowania obiekty 3D dla każdego Ucz się więcej. Zaloguj się Utwórz konto. Zaloguj się Przystąpić. Oferta specjalna! Poza : : : Tylko wybrane elementy. Zobacz więcej ofert w Moje konto. Use arrow keys.
Other identifiers. This provision requires SDSs and information on hazardous substances i. Substances for which industrial accident prevention and reporting requirements have been established.
.
Acetylene or C2H2 is the simplest alkyne and a hydrocarbon that is colorless and has a garlic-like odor. It is highly reactive to atmospheric temperature and lacks oxygen being an unsaturated compound due to the presence of two carbon atoms bonded with a triple bond. As acetylene is reactive, unstable, and lighter than the air, the gas is highly flammable and leads to an explosion. Irrespective of being toxic, acetylene is used for welding purposes as it is flammable. To human beings, this compound is no less than an element of risk as to the existence of it in the atmosphere reduces the level of oxygen. Not only it affects human beings but other living species as well disturbing various natural atmospheric cycles for whom oxygen is an integral component.
C2h2 lewis structure
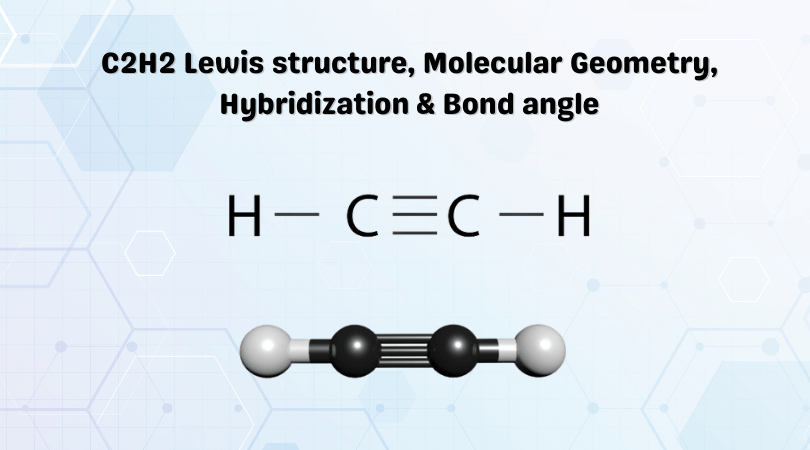
Ethyne or Acetylene is an organic compound used in the synthesis of other hydrocarbon compounds as well as for cutting and welding work. It has a chemical formula of C2H2 and is made up of two atoms of Carbon and two atoms of Hydrogen. To determine its polarity one has to look at its Lewis Structure first to understand the molecular geometry and the shape of the molecule. In the Lewis structure of C2H2 , Carbon atoms take the central position, and the Hydrogen atoms are arranged around it. Both Carbon atoms share one valence electron with one Hydrogen atom by forming a single bond.
Poki fruit
Stereo Microscope. Jul 9, It is possible that a harmonisation is introduced through an amendment to the CLP Regulation. Niniejsza strona używa plików cookies, aby zapewnić optymalne korzystanie z naszych stron internetowych. Uses at industrial sites This substance is used in the following products: pH regulators and water treatment products. Such chemical equations must obey certain rules:. Release to the environment of this substance can occur from industrial use: manufacturing of the substance, as processing aid, formulation of mixtures and as an intermediate step in further manufacturing of another substance use of intermediates. When information is available in all sources, the first two are displayed as a priority. Utwórz zgłoszenie. This list contains hazardous substances for purposes of the Medical Devices Regulation MDR , based on the legislation's Annex I general safety and performance requirements, including for chemical, physical and biological properties. Liczba gwiazdek: 3. ECHA has no public registered data indicating whether or into which articles the substance might have been processed. The physical state of an element relates to the three states of matter, and the precise state for an element is largely determined by the temperature.
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms.
Notice because of the balancing of charges, 1 mole of each of the reactants produces 2 moles of NaCl. The number of atoms of each element in a molecule determines the ratio of the elements in the molecule and is referred to as the stoichiometry of the molecule. Previous page. The products involve 3Cl, while the reactants involve only 1Cl [?? Translated names. Dowiedz się więcej, jak działają opinie klientów w serwisie Amazon. The information is aggregated from the data coming from REACH substance registrations provided by industry. Dodaj do koszyka. The overall ionic charges must be the same on each side of the equation. This information has not been reviewed or verified by ECHA, and may change without prior notice. Buchner Funnel. While the harmonised list covers many hazardous substances, others not listed may also meet the classification criteria in accordance with the CLP Regulation. Guidance on safe use - recommendations by substance registrant on the proper use of the substance in various situations. Manufacture Release to the environment of this substance can occur from industrial use: manufacturing of the substance, as processing aid, formulation of mixtures and as an intermediate step in further manufacturing of another substance use of intermediates. Harmonised classification and labelling is a legally binding classification and labelling for a substance, agreed at European Community level.

You did not try to look in google.com?
I like it topic