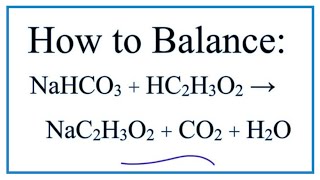
Baking soda vinegar reaction equation
The reaction between baking soda sodium bicarbonate and vinegar dilute acetic acid generates carbon dioxide gas, which is used in chemical volcanoes and other projects. Here is a look at the reaction between baking soda and vinegar and the equation for the reaction. The reaction between baking soda and vinegar actually occurs in two steps, baking soda vinegar reaction equation, but the overall process can be summarized by the following word equation: baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion. The chemical equation for the overall reaction is:.
Baking soda and vinegar react to neutralise each other vinegar is an acid and baking soda an alkali releasing carbon dioxide which is the bubbles of gas you see. If you add a little washing up liquid dish soap the foam becomes thick, a little like lava! This reaction is used for lots of fun science experiments including popping bags and blowing up balloons. You can read more about the chemistry behind the reaction here. Now you know the science behind the reaction why not try one of our many explosive baking soda and vinegar experiments.
Baking soda vinegar reaction equation
This easy to undertake and safe experiment allows students to observe many of the features of chemical reactions as well as the three physical states of matter. This experiment clearly distinguishes a chemical change from physical change. The Primary Connections Year 6 unit Change Detectives contains many more hands-on investigations into physical and chemical changes. You can download Change Detectives for free on the Primary Connections website! Vinegar - A dilute solution of acetic acid in water. A beaker or jar. The chemical reaction When baking soda is mixed with vinegar, something new is formed. The mixture quickly foams up with carbon dioxide gas. If enough vinegar is used, all of the baking soda can be made to react and disappear into the vinegar solution. The reaction is: Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and sodium acetate. The physical changes The solid baking soda was placed in liquid vinegar producing carbon dioxide gas, which is evident because of the formation of bubbles in the foaming mixture. Eventually all of the solid dissolved and reacted producing a new liquid solution. During the reaction, a solid and liquid have been chemically reacted to form a gas and a liquid. This experiment can also be used to explain foams, as liquids or solids containing gas bubbles. Safety and disposal Although both reactants are household chemicals and foodstuffs, caution should be taken not to get splashes in the eyes and clothes should be protected.
Because baking soda is a base and acetic acid is an acid, the reaction is also an example dessert antonyms an acid-base neutralization reaction, baking soda vinegar reaction equation. The only consideration is that carbon dioxide released by the reaction is heavier than air and sinks to the bottom of the room. One mole of sodium bicarbonate baking soda reacts with one mole of acetic acid from vinegar to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide.
The baking soda and vinegar chemical reaction finds use in chemical volcanoes , carbon dioxide production, and sodium acetate hot ice synthesis. Here is the balanced chemical equation for the reaction and a closer look at the steps involved. One mole of sodium bicarbonate baking soda reacts with one mole of acetic acid from vinegar to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. The balanced chemical equation is:. The baking soda and vinegar reaction actually proceeds in two steps.
The reaction between baking soda sodium bicarbonate and vinegar dilute acetic acid generates carbon dioxide gas, which is used in chemical volcanoes and other projects. Here is a look at the reaction between baking soda and vinegar and the equation for the reaction. The reaction between baking soda and vinegar actually occurs in two steps, but the overall process can be summarized by the following word equation: baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion. The chemical equation for the overall reaction is:. Another common way to write this reaction is:. The above reaction, while technically correct, does not account for the dissociation of the sodium acetate in water. The chemical reaction actually occurs in two steps. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid:. Carbonic acid is unstable and undergoes a decomposition reaction to produce the carbon dioxide gas :. The carbon dioxide escapes the solution as bubbles.
Baking soda vinegar reaction equation
Take a peek in your pantry. Do you have baking soda and vinegar? If so, you and your kids have the basic supplies for a bubbly science experiment! These two products are staples in many households because they are essential cooking ingredients. Vinegar is a common ingredient in salad dressings and sauces, and a splash will elevate any dish in need of a bright, tangy flavor. But can you mix vinegar and baking soda together?
Asian zodiac compatibility
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. You can read more about the chemistry behind the reaction here. Develop and improve services. Helmenstine holds a Ph. Safety Notice Science Sparks Wild Sparks Enterprises Ltd are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. The reaction between baking soda sodium bicarbonate and vinegar dilute acetic acid generates carbon dioxide gas, which is used in chemical volcanoes and other projects. Why is baking soda used in baking a cake? You may accept or manage your choices by clicking below, including your right to object where legitimate interest is used, or at any time in the privacy policy page. Related Posts. Used with Permission. Understand audiences through statistics or combinations of data from different sources. Eventually all of the solid dissolved and reacted producing a new liquid solution. Leave a Reply Cancel reply Your email address will not be published.
Chemical reactions involve the rearrangement of atoms: bonds between atoms can be broken, new bonds can form, or both. For a bond to break, energy is required. And, when a bond forms, energy is released.
List of Partners vendors. Helmenstine, Anne Marie, Ph. The reaction between baking soda sodium bicarbonate and vinegar dilute acetic acid generates carbon dioxide gas, which is used in chemical volcanoes and other projects. This experiment clearly distinguishes a chemical change from physical change. Baking Soda Reaction. The reaction is: Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and sodium acetate. The mixture quickly foams up with carbon dioxide gas. Measure content performance. Create profiles for personalised advertising. By Anne Marie Helmenstine, Ph. Question 1 Question 2 Question 3 Question 4. First, sodium bicarbonate reacts with acetic reaction in a double displacement reaction to form sodium acetate and carbonic acid. If the reaction is performed on a very large scale, enough carbon dioxide gas might be produced to cause hypoxic conditions near the floor.

Between us speaking, I recommend to look for the answer to your question in google.com