9 bbn structure
From Wikimedia Commons, the free media repository. File information. Structured data. Captions Captions English Add a one-line explanation of what this file represents.
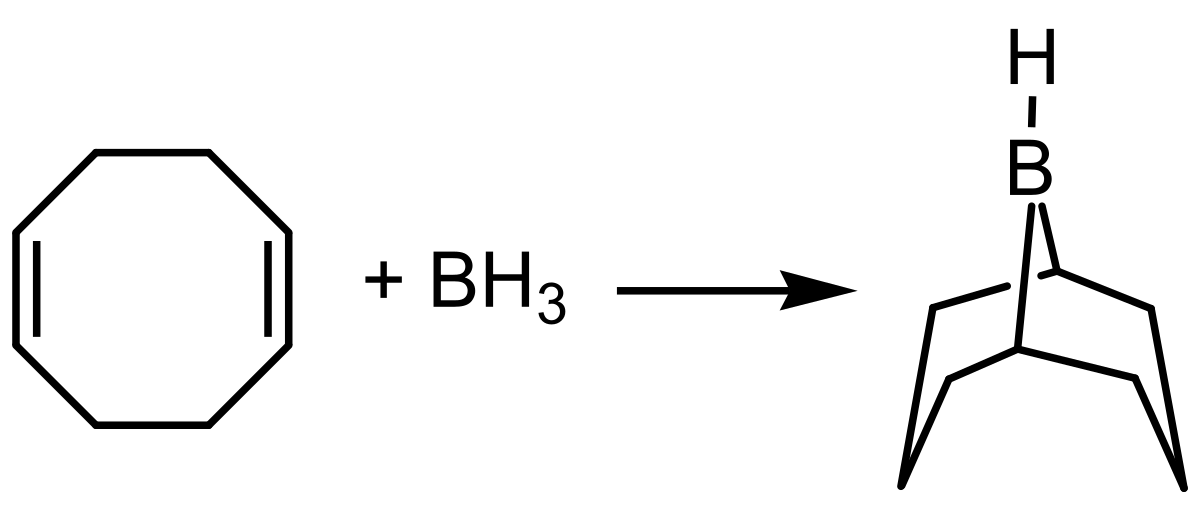
This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substrates. The compound is commercially available as a solution in tetrahydrofuran and as a solid. Its highly regioselective addition on alkenes allows the preparation of terminal alcohols by subsequent oxidative cleavage with H 2 O 2 in aqueous KOH. The steric demand of 9-BBN greatly suppresses the formation of the 2-substituted isomer compared to the use of borane.
9 bbn structure
.
Download as PDF Printable version.
.
Ramos uclm. The commercial 9-borabicyclo[3. Stoichiometric reactions, kinetic studies, and DFT calculations have allowed us to propose a plausible mechanism involving a heterocyclic amidinate intermediate with a three center-two electron B—H—B bond. Ramos, A. Carrillo-Hermosilla, R. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Read more about how to correctly acknowledge RSC content. Fetching data from CrossRef.
9 bbn structure
The hydroboration-oxidation of alkynes is similar to the reaction with alkenes. However, there is one important difference. The alkyne has two pi bonds and both are capable of reacting with borane BH 3. To limit the reactivity to only one of the pi bonds within the alkyne, a dialkyl borane reagent R 2 BH is used. Replacing two of the hydrogens on the borane with alkyl groups also creates steric hindrance so that the hydroboration reaction produces the regioselective,. Disiamylborane Sia 2 BH and 9-borabicyclo[3. Their structures are shown below.
Is fairy power spray still available in uk
Signal word. This image of a simple structural formula is ineligible for copyright and therefore in the public domain , because it consists entirely of information that is common property and contains no original authorship. Structured data Items portrayed in this file depicts. Wikimedia Commons. Download as PDF Printable version. Public domain Public domain false false. The steric demand of 9-BBN greatly suppresses the formation of the 2-substituted isomer compared to the use of borane. English: Structural formula of monomeric 9-borabicyclo[3. N verify what is Y N? Deutsch: Strukturformel von monomerem 9-Borabicyclo[3. Other names Borabicyclononane Banana borane. Views View Edit History.
An especially valuable group of intermediates can be prepared by addition of an compound to carbon-carbon double or triple bonds:.
The substitution of any brackets is due to technical restrictions. Wikimedia Commons. English: Structural formula of monomeric 9-borabicyclo[3. The steric demand of 9-BBN greatly suppresses the formation of the 2-substituted isomer compared to the use of borane. Its highly regioselective addition on alkenes allows the preparation of terminal alcohols by subsequent oxidative cleavage with H 2 O 2 in aqueous KOH. Scientific American Blog Network. PubChem CID. N verify what is Y N? Namespaces File Discussion. MIME type. Nature America, Inc.

There are also other lacks
The authoritative point of view, it is tempting
I like this phrase :)