When calcium carbonate is heated
Byju's Answer.
In association with Nuffield Foundation. Calcium carbonate is strongly heated until it undergoes thermal decomposition to form calcium oxide and carbon dioxide. This experiment can be carried out conveniently in groups of two or three and takes about 40—45 minutes. Keep an eye on less mature students who might be tempted to suck rather than blow through the filtrate. This set of experiments involves a variety of important reactions and types of reactions, with several references to industrial processes.
When calcium carbonate is heated
.
Add universal indicator. The chalk should be seen to crumble slightly. Only registered users can comment on this article.
.
Calcium carbonate, calcium oxide and calcium hydroxide are all made from limestone and have important applications so it is important to know how they are made. Calcium carbonate is found naturally in limestone close limestone A type of sedimentary rock. When limestone is heated strongly, the calcium carbonate it contains absorbs heat endothermic close endothermic Reaction in which energy is taken in. This is indicated by an orange glow as the limestone is heated. Calcium oxide also known as quicklime is a key ingredient in the making of cement and is also used to make certain types of plaster. Calcium oxide reacts with a few drops of water to form calcium hydroxide, which is an alkali close alkali A base which is soluble in water.
When calcium carbonate is heated
Calcium carbonate is the principal mineral component of limestone. Its chemical and physical properties lie behind the modern-day uses of limestone as well as the unique limestone landscapes of the countryside. The principal mineral component of limestone is a crystalline form of calcium carbonate known as calcite. Although calcite crystals belong to the trigonal crystal system, shown below, a wide variety of crystal shapes are found. Single calcite crystals display an optical property called birefringence double refraction. This strong birefringence causes objects viewed through a clear piece of calcite to appear doubled. Another mineral form of calcium carbonate is called aragonite. Its crystal lattice differs from that of calcite, resulting in a different crystal shape — an orthorhombic system with needle-shaped crystals. Calcium carbonate is unusual in that its solubility increases as the temperature of the water decreases. The increased solubility of calcium carbonate in rainwater saturated with carbon dioxide is the driving force behind the erosion of limestone rocks, leading to the formation over long periods of time of caverns, caves, stalagmites and stalactites.
Usdt to aud converter
Calcium carbonate decomposes into calcium oxide and carbon dioxide. This is what happens in lime kilns where limestone calcium carbonate is heated to form lime calcium oxide. Add 2—3 drops of water. More crumbling, steam given off, evidence that mixture has become hot. Resource Paracetamol book Using thin-layer chromatography to investigate the reactions In this activity you investigate the purity and identity of your laboratory prepared samples of nitrophenol or paracetamol using thin-layer chromatography. Is this reaction reversible or irreversible? You're not signed in. Use Practical experiments. Calcium carbonate is strongly heated until it undergoes thermal decomposition to form calcium oxide and carbon dioxide. Resource Paracetamol book The preparation of paracetamol The first of three steps, in practical experiments, that show learners how to prepare paracetamol. More from Experiments. According to law of conservation of mass, if 5.
In association with Nuffield Foundation. Calcium carbonate is strongly heated until it undergoes thermal decomposition to form calcium oxide and carbon dioxide. This experiment can be carried out conveniently in groups of two or three and takes about 40—45 minutes.
This experiment can be carried out conveniently in groups of two or three and takes about 40—45 minutes. Add 2—3 drops of water. Calcium carbonate decomposes into calcium oxide and carbon dioxide. Only registered users can comment on this article. In association with Nuffield Foundation. Related articles. Resource Paracetamol book The preparation of paracetamol The first of three steps, in practical experiments, that show learners how to prepare paracetamol. Site powered by Webvision Cloud. Sign in Register. Add 10 cm 3 more water. Calcium carbonate is strongly heated until it undergoes thermal decomposition to form calcium oxide and carbon dioxide.

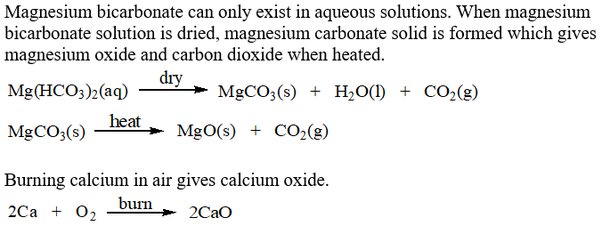
Rather valuable piece
I consider, that you are not right. Let's discuss. Write to me in PM, we will communicate.