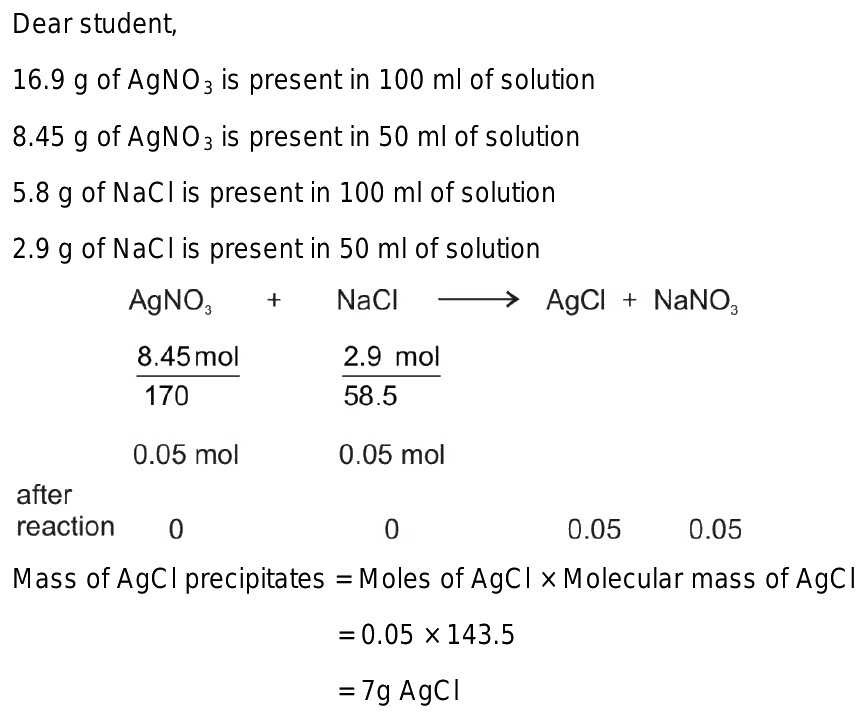
What is the mass of precipitate formed
Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate.
We endeavor to keep you informed and help you choose the right Career path. When you look back in life , this app would have played a huge role in laying the foundation of your career decisions. Found everything I wanted and it solved all of my queries for which I was searching a lot A must visit No need to find colleges in other sites, this is the best site in India to know about any colleges in India.
What is the mass of precipitate formed
Q: What mass of precipitate in g is formed when Q: How many grams of AgCi will be formed when 60 mL of 0. Q: A Q: How many ters of a 0. Q: How many moles of precipitate will be formed when Q: One of the active ingredients in stain removers is sodium percarbonate Na2CO3 , and a typical stain…. Q: Calculate the Molarity when A: Molarity can be defined as the number of moles of solute dissolved per litre of solution. It is a…. A: This can be solved by balancing the equation. Q: How many grams of solid Ni OH 2 are formed when
More Report.
Questions » Accounting » Auditing » What mass of precipitate in g is formed when Questions Courses. What mass of precipitate in g is formed when May 15 AM. Subhash P answered on May 17, Step 2 of 5: Precipitation reaction are reaction where two soluble salts reacts to form an insoluble salt which Do you need an answer to a question different from the above?
Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. Because not all aqueous reactions form precipitates, one must consult the solubility rules before determining the state of the products and writing a net ionic equation. The ability to predict these reactions allows scientists to determine which ions are present in a solution, and allows industries to form chemicals by extracting components from these reactions. Precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. The determining factors of the formation of a precipitate can vary. Some reactions depend on temperature, such as solutions used for buffers, whereas others are dependent only on solution concentration. The solids produced in precipitate reactions are crystalline solids, and can be suspended throughout the liquid or fall to the bottom of the solution.
What is the mass of precipitate formed
A chemist combines mL of a 0. How many grams of precipitate form? By solubility rules, is soluble in water and is not. Our new reaction is:. Now we perform the same calculation beginning with :. The limiting reagent is and this reaction produces A chemist boils off the water from mL of a 0. What is the mass of the remaining solid? First, you must recognize that the chemical formula for sodium hydroxide is. The mass of the boiled solution is.
Tough mudder 2023 brisbane
Titrations: Strong Acid-Strong Base. Write a balanced chemical equation for the reaction that… A: Barium hydroxide is a base and nitric acid is acid Reaction between these two is a neutralization…. Lawrence S. Condensed Formula. The solubility by which we usually mean the molar solubility of a solid is expressed as the concentration of the "dissolved solid" in a saturated solution. Chemical Reactions 4h 8m. Hydroxide precipitation is probably the most widely used industrial precipitation process in which metal hydroxides are formed by adding calcium hydroxide slaked lime or sodium hydroxide caustic soda as precipitant. Chemistry: Principles and Reactions 8th Edition. The audit senior has asked you to perform analytical pro-cedures to obtain substantive evidence on the reasonableness of recorded depreciation expense of the delivery equipment of a client. Naming Aldehydes.
Chemistry Glossary Definition of Precipitate. In chemistry, to precipitate is to form an insoluble compound either by reacting two salts or by changing the temperature to affect the solubility of the compound. Precipitation may indicate a chemical reaction has occurred, but it may also occur if a solute concentration exceeds its solubility.
Titrations: Strong Acid-Strong Base. Constant-Pressure Calorimetry. Intro to Chemical Equilibrium. World of Chemistry, 3rd edition. Quantum Numbers: Nodes. This means that both the products are aqueous i. HCl content… A:. Reaction Mechanism. Solutions: Mass Percent. Precipitation: Ksp vs Q. Q: Many metal ions are precipitated from solution by the sulfide ion. Weak Titrate-Strong Titrant Curves. Q: What is the molarity of a solution in which Edit Comment. Verified Solution.

0 thoughts on “What is the mass of precipitate formed”