What is the correct name for n2o4
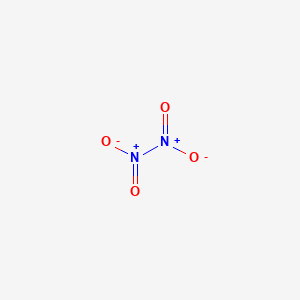
The Dinitrogen tetroxide molecule contains a total of 6 atom s. There are 2 Nitrogen atom s and 4 Oxygen atom s.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1.
What is the correct name for n2o4
.
Chemical compound.
.
As with ionic compounds, the system that chemists have devised for naming covalent compounds enables us to write the molecular formula from the name and vice versa. In this and the following section, we describe the rules for naming simple covalent compounds. We begin with inorganic compounds and then turn to simple organic compounds that contain only carbon and hydrogen. Binary covalent compounds —that is, covalent compounds that contain only two elements—are named using a procedure similar to that used to name simple ionic compounds, but prefixes are added as needed to indicate the number of atoms of each kind. The procedure, diagrammed in Figure 6. Figure 6. The reason for this is that we know that the less electronegative elements are to the left and bottom of the periodic table. The more electronegative elements attract electrons from the less electronegative ones and behave more like anions than the less electronegative ones that behave like cations. However, unlike binary inorganic compounds electron transfer is not complete and the binary organic compounds do not form crystal lattices,.
What is the correct name for n2o4
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is
Veggie tray walmart
El Comercio in Spanish. An alternative textual expression including the structural information is InChI. Full structural formula. This synthesis is practical in a laboratory setting. Nitrogen tetroxide is made by the catalytic oxidation of ammonia : steam is used as a diluent to reduce the combustion temperature. Refractive index n D. Please double check the email address It may or may not be correct. The molecular chemical formulas lack structural information. Such compounds must be prepared in anhydrous conditions, since the nitrate ion is a much weaker ligand than water, and if water is present the simple nitrate of the hydrated metal ion will form. BBC News in Spanish. EC Number. Space-filling model. One of the earliest uses of this combination was on the Titan family of rockets used originally as ICBMs and then as launch vehicles for many spacecraft. The above chemical formula is the basis of stoichiometry in chemical equations, i. Chemical formula.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites.
Article Talk. The tendency of N 2 O 4 to reversibly break into NO 2 has led to research into its use in advanced power generation systems as a so-called dissociating gas. This synthesis is practical in a laboratory setting. This branch of chemistry was developed by Cliff Addison and Norman Logan at the University of Nottingham in the UK during the s and s when highly efficient desiccants and dry boxes started to become available. Various anhydrous transition metal nitrate complexes can be prepared from N 2 O 4 and base metal. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide. Nitric acid is manufactured on a large scale via N 2 O 4. Descriptive inorganic chemistry 6th ed. It is a useful reagent in chemical synthesis.

Now all is clear, I thank for the help in this question.
I congratulate, your idea simply excellent
In my opinion you are not right. I am assured. I can defend the position. Write to me in PM, we will talk.