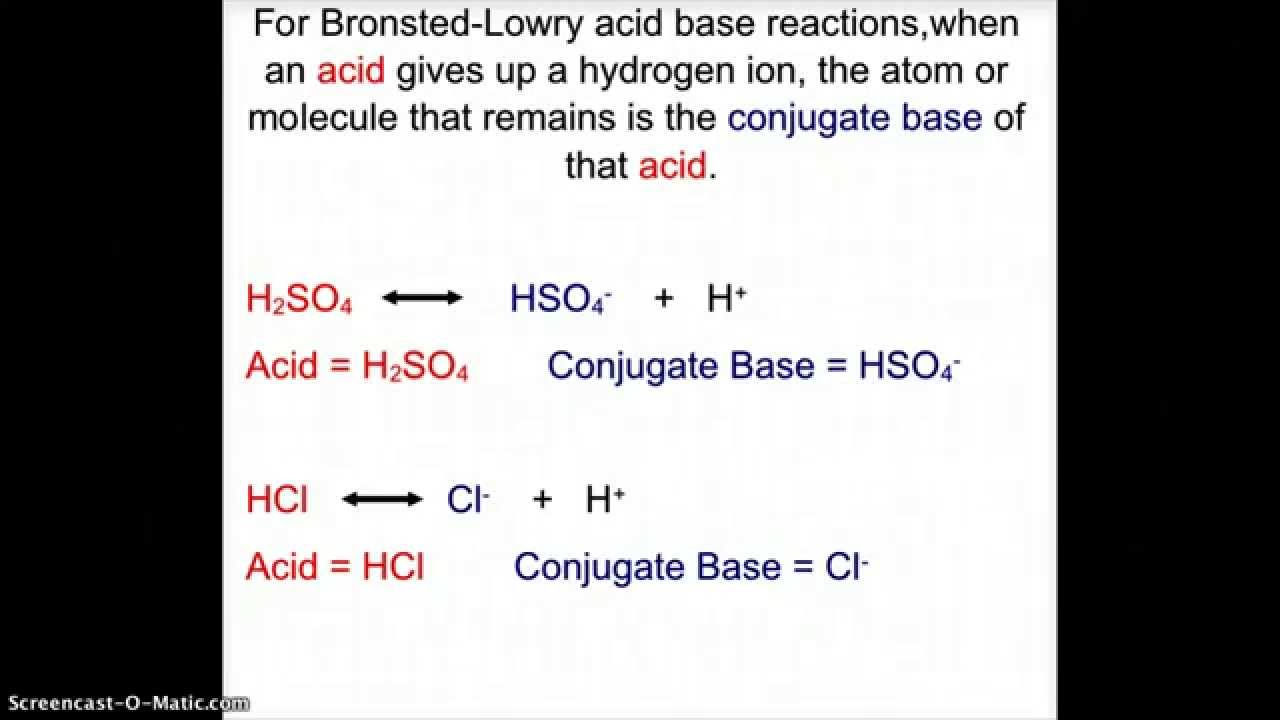
What is the conjugate base of h2so4
Identify the acid, base, conjugate acid and conjugate base in the following reaction. HSO4" aq ….
What is the conjugate of H 2 SO 4? A non-metallic element is converted into a compound X after a series of reactions. A little amount of X when tested with blue litmus turns to red. X on complete reaction with another compound Y gave the product which did not respond to litmus test. Identify the correct sequence of the reactions.
What is the conjugate base of h2so4
Wiki User. They are the products of an acid-base reaction by the Bronsted-Lowry definition. Conjugate base. HSO4 -. Nope, itsHSO H2SO4 is already a strong acid. If you mean what is the conjugate base, then the answer is HSO The conjugate base of a weak acid is always a strong base. You mean sulfuric acid. The conjugate base of HF is the fluoride ion F-.
HCN 4. Q: Identify the acid, base, conjugate acid, and conjugate base in the following reactions.
.
See this Socratic answer. A conjugate base contains one less H atom and one more - charge than the acid that formed it. Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions. It has one less H atom and one more — charge. Acid strength is determined by the amount of that acid that actually ionizes. All other acids are weak acids and ionize to a much lesser amount. All acids have a conjugate base. All bases have a conjugate acid. This is most easily seen when they dissociate in water:.
What is the conjugate base of h2so4
Through examples found in the sections on acids and bases proton-transfer processes are broken into two hypothetical steps: 1 donation of a proton by an acid, and 2 acceptance of a proton by a base. Water served as the base in the acid example and as the acid in the base example [ amphiprotic ]. The hypothetical steps are useful because they make it easy to see what species is left after an acid donated a proton and what species is formed when a base accepted a proton. We shall use hypothetical steps or half-equations in this section, but you should bear in mind that free protons never actually exist in aqueous solution. Suppose we first consider a weak acid , the ammonium ion. When it donates a proton to any other species, we can write the half-equation:. The submicroscopic representations below show the donation of the proton of ammonium. The removal of this proton results in NH 3 , which is easily seen at the submicroscopic level. But NH 3 is one of the compounds we know as a weak base.
Videos colegialas calientes
Publisher: John C. Find more answers. Q: What is the conjugate base of H2SO4? Q: What is the conjugate base of phosphoric acid? Nope, itsHSO Continue Learning about Chemistry. The conjugate base of H3O' hydronium ion is a strong acid, strong…. Reger, Scott R. When a base is capable of accepting a proton and there is the removal of a proton from the acid is known as conjugate base. A strong acid will form a weak….
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases.
Zumdahl, Donald J. A: The acid given is phosphoric acid i. Ka for… A: The more ka value of acid the more acidic and the conjugatebase of that strong acid is weakest…. John C. A: Explanation to the correct answer is given below. A acid B base C conjugate acid… A:. Oxyacids of Phosphorus. Expert Solution. Wiki User. What is the base of H2SO4? Knowledge Booster. What is the formula of the conjugate acid for base rm SO 42? Q: Identify the acid associated with each conjugate base. A non-metallic element is converted into a compound X after a series of reactions.

It is an amusing phrase
It agree, it is the remarkable answer
I congratulate, it seems brilliant idea to me is