Velocity of an electron in nth orbit
Angular momentum. Speed of moving electron in the orbit. Planck's constant.
For electron moving in n t h orbit of the atom , the angular velocity is proportional to:. If an electron is moving in nth orbit of H-atom, then its velocity is-. In Bohr's orbit , kinetic energy of an electron in the n t h orbit of an atom in terms of angular momentum is propotional to. The magnetic field induction produced at the centre of orbit due to an electron revolving in n t h orbit of hydrogen atom is proportional to. The angular speed of the electron in the n t h Bohr orbit of the hydrogen atom is proportional to.
Velocity of an electron in nth orbit
The velocity of the electron in the first Bohr's orbit is 2. The linear velocity of electron in third orbit is. The velocity of electrons in the ground state of H- atom is 2. The velocity of an electron in the first Bohr orbit of hydrogen atom is 2. Its velocity in the second orbit would be. If the speed of electron in the first bohr orbit of hydrogen atom is x then the speed of the electron in the third Bohr orbit of hydrogen is. The velocity of an electron in the first orbit of H atom is v. The velocity of an electron in the first orbit of H-atom is v. The velocity of an electron in the 2nd orbit. The circumference of the first Bohr orbit in H atom is 3. What is the velocity of the electron of this orbit? The velocity of the electron in the first Bohr orbit of H-atoms is 2. If the speed of electron in the first bohr orbit of hydrogen atom is x Calculate the velocity of an electron in the first Bohr orbit of a hyd In first Bohr orbit of hydrogen atom, the velocity of electron would b
Assertion: The de Broglie wavelength of an electron in n t h Bohr orbit of hydrogen is inversely proportional to the square of quantum number n.
From the above explanation, we can see that the speed on n th electron in a Hydrogen atom can be expressed as. Get Started. English Hindi. This question was previously asked in. Start Now. Concept: For H-atom where n is an integer, r n is the radius of n th possible orbit and v n is the speed of moving electron in the n th orbit We know that if a particle is bound in an atom, it is because of the balanced force between columbic force of attraction between protons and electrons and centripetal force bound by H-atoms nucleus i. Learn today!
Are you looking for an electron speed calculator to estimate both the classical and relativistic speed of an electron? Be our guest! In this calculator, you can not only estimate the relativistic and non-relativistic velocities of an electron under a given accelerating potential, but you will also learn:. Last but not least, you'll learn answers to some interesting questions, like "Do electrons move near the speed of light? Atoms consist of three basic components: electrons, protons, and neutrons. An electron has a mass of 9. Acceleration of a particle in an electric field is possible if it carries a charge. Therefore, electromagnetic fields accelerate electrons. If you know the value of the electric field's potential, you can calculate the speed of an electron moving under its influence using the equation for kinetic energy. Read on to learn how to calculate the speed of an electron accelerated by an electric field.
Velocity of an electron in nth orbit
The model has a special place in the history of physics because it introduced an early quantum theory, which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. When we use a prism to analyze white light coming from the sun, several dark lines in the solar spectrum are observed Figure 6. Solar absorption lines are called Fraunhofer lines after Joseph von Fraunhofer , who accurately measured their wavelengths. During —, Gustav Kirchhoff and Robert Bunsen discovered that for the various chemical elements, the line emission spectrum of an element exactly matches its line absorption spectrum. The difference between the absorption spectrum and the emission spectrum is explained in Figure 6. An absorption spectrum is observed when light passes through a gas. This spectrum appears as black lines that occur only at certain wavelengths on the background of the continuous spectrum of white light Figure 6. The missing wavelengths tell us which wavelengths of the radiation are absorbed by the gas.
Closest loves near me
Slope of distance-time graph gives:. In uniform circular motion the following quantities remain constant-. More Than Just We take learning seriously. Matter Waves. How much interest did it earn in the first year? Sign Up. The velocity of an electron in the first Bohr orbit of hydrogen atom i The velocity of an electron in the nth orbit of a hydrogen atom is 2. Competition Change. Bohr's quantization condition is:. Upgrade to add a comment. Com Master of Commerce M. Find the radius of the first orbit of hydrogen atom. Suggested Textbook. In Bohr's orbit , kinetic energy of an electron in the n t h orbit of an atom in terms of angular momentum is propotional to.
Bohr's model of the atom was valuable in demonstrating how electrons were capable of absorbing and releasing energy, and how atomic emission spectra were created.
An X ray tube is operated at an accelerating potential of 40 kV. Hospitality and Tourism Change. If the speed of electron in the first bohr orbit of hydrogen atom is x then the speed of the electron in the third Bohr orbit of hydrogen is. Sign Up Free. Phone Number. Linear momentum of an electron in Bohr obrit of H-atom principal quantum number n is proporational to. Angular momentum. Add To Playlist Hmmm, doesn't seem like you have any playlists. The velocity of the electron in the first Bohr orbit of H-atoms is 2. Try Numerade free for 7 days View This Answer. If the stopping potential for tungsten is measured to be 1. Please add your first playlist.

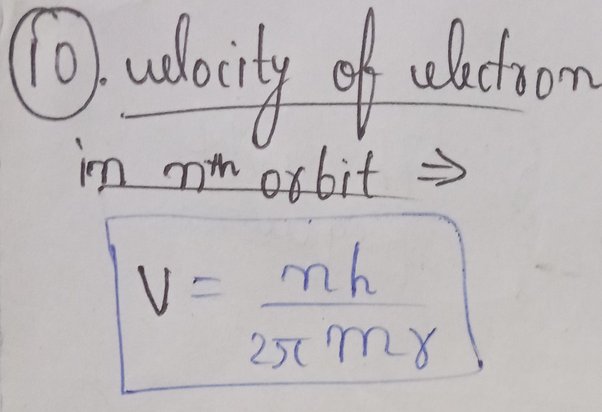
It yet did not get.
This theme is simply matchless :), it is very interesting to me)))
And you so tried?