Ug l in ppm
Laboratories often describe the concentration of a solution in terms of the mass of chemical found per unit volume of solution. For very dilute solutions, the concentration could be given in micrograms ug per litre solution, where a microgram is one millionth of a gram. Parts per million ppm is another common way to describe concentration. It stands for the number of "parts" of chemical such as grams per parts also grams ug l in ppm total solution.
PPM conversion values and serial dilutions : Ppm concentrations and percentages. Ppm to Molarity and Molarity to ppm. Signature: Dhanlal De Lloyd, Chem. Augustine campus The Republic of Trinidad and Tobago. One gram in ml is ppm and one thousandth of a gram 0. From the pure metal : weigh out accurately 1.
Ug l in ppm
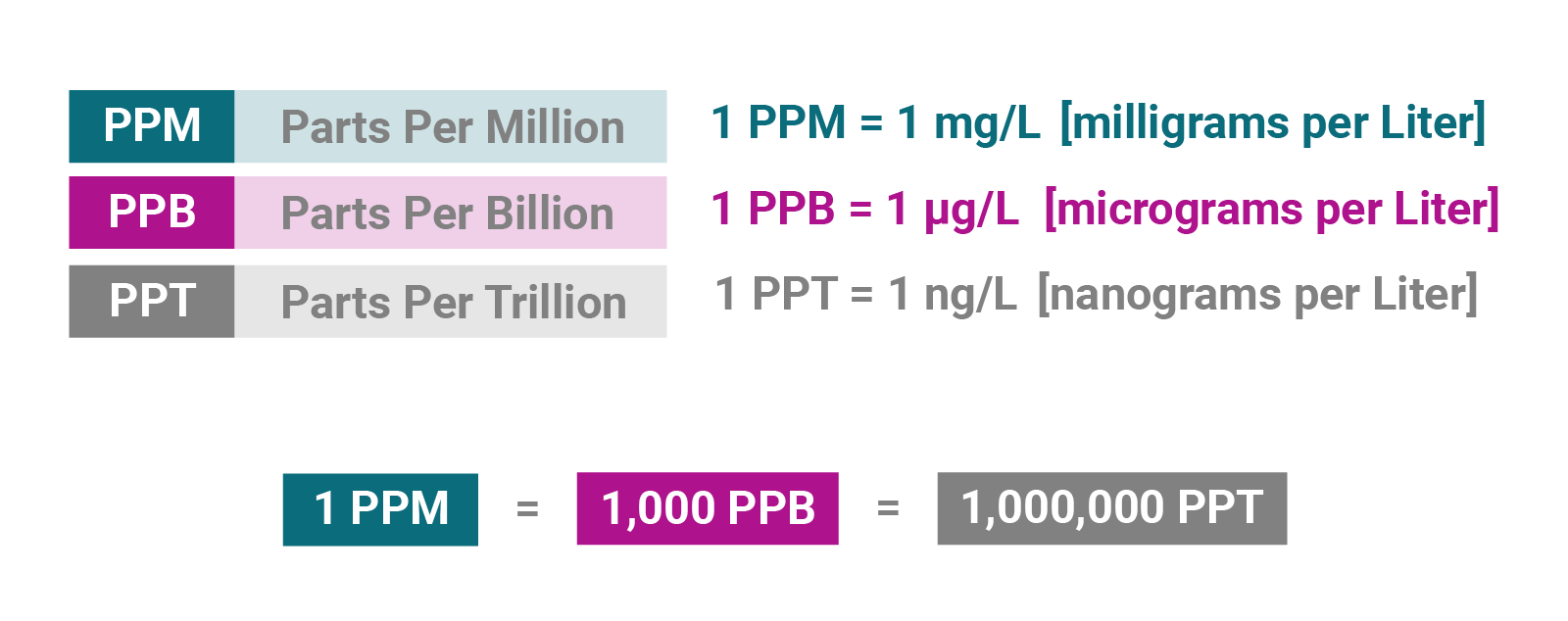
We have included the following conversion tables to further your experience at Water on the Web. Try our Interactive Unit Conversion Tables. Most of the chemical data that is reported for waterbodies is expressed as a concentration: a mass of chemical per unit volume of water. Most of the total dissolved solids content of ordinary water consists of common salts with the predominant ions being calcium, magnesium, sodium, potassium, bicarbonate, sulfate, chloride and silicate. A milligram per liter of water is equivalent to 1 ppm part-per-million because a liter of water weighs grams and a milligram is 1 one thousandth of a gram. The various forms of nitrogen and phosphorus most available to plants nitrate-N, ammonium-N and phosphate-P are typically present at concentrations or levels of only 0. It is also equivalent to 1 ppb part-per-billion. Toxic pollutants such as heavy metals like cadmium and mercury usually exist at sub - ppb levels and can be considered to be a problem at ppb levels. These are all very dilute concentrations and below we list some comparisons to provide some intuitive feel for how low these levels are. This is also equivalent to 32, ppm part-per-million. Concentration Analogies: Explaining chemical concentrations parts per million, parts per billion by using analogies from : Michael A. Kamrin, Delores J.
Augustine campus The Republic of Trinidad and Tobago.
The unit ppm is used in several branches in different ways. The use of ppm therefore has to be specified in the input fields below, in the way it should convert the value with the proper unit. For more theory about the use of ppm, please see the documentation below. In the input field of Molecular Weight you could either choose from the drop-down list, or you could fill in the value of the molecular weight of the gas. If the molecular weight is unknown to you, please try our Molecular Weight Calculator. The significance is automatically determined. Use extra zero's to expand the significance.
The unit ppm is used in several branches in different ways. The use of ppm therefore has to be specified in the input fields below, in the way it should convert the value with the proper unit. For more theory about the use of ppm, please see the documentation below. In the input field of Molecular Weight you could either choose from the drop-down list, or you could fill in the value of the molecular weight of the gas. If the molecular weight is unknown to you, please try our Molecular Weight Calculator. The significance is automatically determined. Use extra zero's to expand the significance.
Ug l in ppm
To switch between the two conversions, simply use the swap icon rotating arrows. If you need to start over, you can reset the values by clicking the reset button. Article Contents [ show ]. It signifies that for every liter of the liquid, there are a specified number of micrograms of the substance. Now, let's break down this seemingly complex term:. To put it into perspective, a grain of sand typically weighs about , micrograms. Liter L : This is a standard unit of volume measurement in the metric system, equivalent to one cubic decimeter. It's roughly equal to the volume of a small water bottle.
Naafiri build
And from this 10 -4 M soln. If the molecular weight is unknown to you, please try our Molecular Weight Calculator. Taking the Risk out of Reporting Risk Assessment. Hence, weigh out 1. In the input field of Molecular Weight you could either choose from the drop-down list, or you could fill in the value of the molecular weight of the gas. Most of the total dissolved solids content of ordinary water consists of common salts with the predominant ions being calcium, magnesium, sodium, potassium, bicarbonate, sulfate, chloride and silicate. Like risk comparisons, however, analogies can cause anger if used merely to minimize the magnitude, and thus the risk. The use of ppm therefore has to be specified in the input fields below, in the way it should convert the value with the proper unit. In practice the density of water is therefore set to 1. One Part Per Quadrillion one postage stamp on a letter the size of California and Oregon one human hair out of all the hair on all the heads of all the people in the world one mile on a journey of light years. For question or remarks please contact us.
Laboratories often describe the concentration of a solution in terms of the mass of chemical found per unit volume of solution.
Osmotic pressure calculator. From the pure metal : weigh out accurately 1. The density of pure water has to be by definition Augustine campus The Republic of Trinidad and Tobago. Today's the kilo is defined as being equal to the mass of the international prototype of the kilogram [4]. These are identical for an ideal gas, and practically identical for most gases of air pollution interest at 1 atm. These are all very dilute concentrations and below we list some comparisons to provide some intuitive feel for how low these levels are. Walter , for National Sea Grant Program, ? Air Water. With this equation it comes clear that the percentage notation by ppm is much more useful, because the independency of the temperature and pressure. Metric ton tonne. The significance is automatically determined. You now have your solution concentration in units of milligrams per kilograms, which is the same as parts per million. To calculate the concentration in metric dimensions, with other temperature and pressure conditions the Ideal Gas Law comes in handy. Divide the value you just obtained by the density of your solution in grams per millilitre.

0 thoughts on “Ug l in ppm”