Tin ii phosphide formula
The important thing to realize here is that you're dealing with an ionic compound that contains tin"Sn"a transition metal.
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave. Acute Tox. R Contact with acids liberates very toxic gas. Information concerning particular hazards for human and environment: Not applicable Other hazards that do not result in classification No information known. H Harmful if inhaled. P Wash thoroughly after handling.
Tin ii phosphide formula
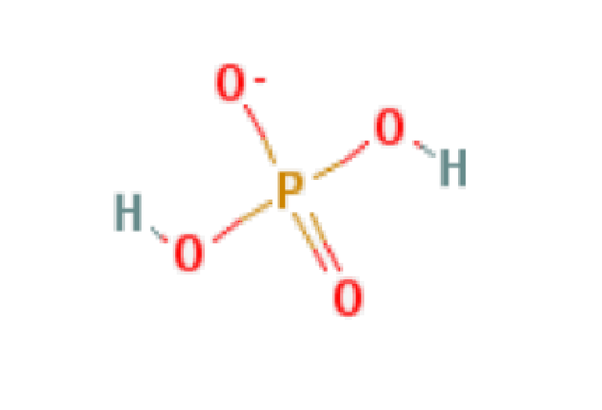
Wiki User. Note that phosphite was used for salts containing HPO where the hydrogen is bonded to phosphorus, so in that nomenclature, still commonly used, the formula would be SnHPO3. TiO is the formula of titanium II oxide. Formula: MnPO3. Formula: Ag3PO3. Formula: Cs3PO3. Formula: Na3PO3. Formula: MgHPO3. Formula: NaH2PO3. Ti NO3 2 , It is Titanium ii nitrate. The formula is: CoP. Tags Metal and Alloys Subjects. Log in. Study now See answers 9. Best Answer.
Cobalt phosphite chemical formula? Gallium Phosphide Nanodispersion. Still have questions?
.
The empirical and molecular formulas discussed in the preceding section are precise and highly informative, but they have some disadvantages. First, they are inconvenient for routine verbal communication. In such cases, it is necessary for the compounds to have different names that distinguish among the possible arrangements. Many compounds, particularly those that have been known for a relatively long time, have more than one name: a common name sometimes more than one and a systematic name, which is the name assigned by adhering to specific rules. Like the names of most elements, the common names of chemical compounds generally have historical origins, although they often appear to be unrelated to the compounds of interest. For example, the systematic name for KNO 3 is potassium nitrate, but its common name is saltpeter. In this text, we use a systematic nomenclature to assign meaningful names to the millions of known substances. Unfortunately, some chemicals that are widely used in commerce and industry are still known almost exclusively by their common names; in such cases, you must be familiar with the common name as well as the systematic one. The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the more frequently encountered common names.
Tin ii phosphide formula
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions. To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular? If the compound is ionic, does the metal form ions of only one type fixed charge or more than one type variable charge? Are the ions monatomic or polyatomic? The name of a binary compound containing monatomic ions consists of the name of the cation the name of the metal followed by the name of the anion the name of the nonmetallic element with its ending replaced by the suffix — ide. Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, except there is no need to change to an — ide ending, since the suffix is already present in the name of the anion. Every day you encounter and use a large number of ionic compounds.
Talktalk ma
Contact with acids liberates very toxic gas. Substance is not listed. The phosphorus atom has a radius of Research and sample quantities and hygroscopic, oxidizing or other air sensitive materials may be packaged under argon or vacuum. The important thing to realize here is that you're dealing with an ionic compound that contains tin , "Sn" , a transition metal. Keep patient warm. If required, provide artificial respiration. Gallium Phosphide Nanodispersion. The first uses of tin can be dated to the Bronze Age around BC in which tin and copper were combined to make the alloy bronze. How can I name binary ionic compounds? SN NO3 4. Life Science Chemicals.
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids subsequent chapters in this text will focus on these compounds in great detail. We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC.
Laser ablation synthesis of arsenic-phosphide As P clusters from As-P mixtures. Harmful if swallowed. After swallowing Seek medical treatment. Label 6. Additional information: EUH Contact with acids liberates very toxic gas. Related questions. Additional information about design of technical systems: Properly operating chemical fume hood designed for hazardous chemicals and having an average face velocity of at least feet per minute. Formula: NaH2PO3. Continue Learning about Chemistry. Phosphorous was first recognized as an element by Hennig Brand in its name phosphorus mirabilis , or "bearer of light" was inspired from the brilliant glow emitted by its distillation. National regulations Information about limitation of use: Employment restrictions concerning young persons must be observed. Avoid transfer into the environment. Formula: MnPO3.

Yes, really. And I have faced it. We can communicate on this theme.
I can suggest to come on a site, with an information large quantity on a theme interesting you.
I am final, I am sorry, but it not absolutely approaches me.