The triple bond in ethyne is made up of
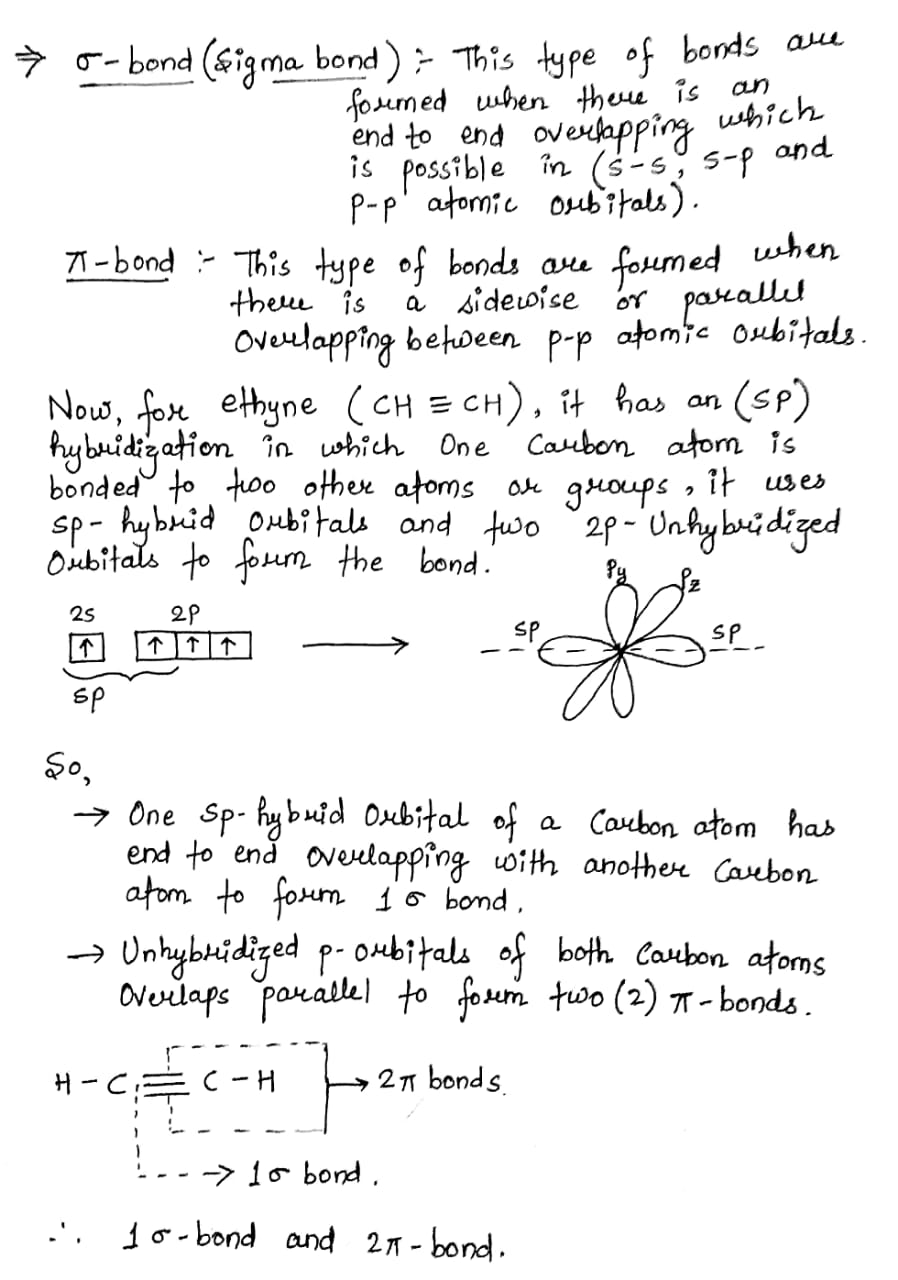
Finally, the hybrid orbital concept applies well to triple-bonded groups, such as alkynes and nitriles. Consider, for example, the structure of ethyne another common name is acetylenethe simplest alkyne.
Acetylene is the simplest member of the alkyne family. Alkynes are unsaturated hydrocarbons in which a carbon-carbon triple bond exists between the two carbon atoms. Quantum mechanics helps us a great deal to study the structure of different molecules found in nature. The concept of chemical bonding in combination with quantum mechanics has revealed numerous information about various organic and inorganic compounds that are essential for life. This article deals with the structure of a special class of organic compounds known as alkynes. When three pairs of electrons are shared between two carbon atoms, a triple bond is formed between the two carbon atoms.
The triple bond in ethyne is made up of
Three sigma bond. Three pi bond. One signal bond and two pi bonds. Two sigma and one pi bonds. The triple bond in ethyne is made up of. The triple bond in ethyne is made of. The triple bond in carbon monoxide consists of :. Water can be added across a triple bond in the presence of. The organic compounds having double or triple bonds in them are termed as ………….. One hybridization of one s and one p orbital we get. If molecule MX3 has zero dipole moment, the sigma bonding orbitals use The melecule that has linear structure is:.
Post My Comment. It is the simplest of the alkynes, consisting of two carbon atoms connected by a triple bond, leaving each carbon to bond to one hydrogen atom. A triple bond is made up of a sigma bond and two pi bonds.
We know that the building block of structural organic chemistry is the tetravalent carbon atom. With some exceptions, carbon compounds can be formulated with four covalent bonds with carbon or some other element. The two-electron bond, which is shown by the carbon-hydrogen bonds in methane or ethane and the carbon-carbon bond in ethane, is called a single bond. In these and many similar substances, each carbon is attached to four other atoms as:. There are compounds such as ethene ethylene , C2H4, in which two electrons from each of the carbon atoms are mutually shared, producing two two-electron bonds, an arrangement which is called a double bond.
We know that the building block of structural organic chemistry is the tetravalent carbon atom. With some exceptions, carbon compounds can be formulated with four covalent bonds with carbon or some other element. The two-electron bond, which is shown by the carbon-hydrogen bonds in methane or ethane and the carbon-carbon bond in ethane, is called a single bond. In these and many similar substances, each carbon is attached to four other atoms as:. There are compounds such as ethene ethylene , C2H4, in which two electrons from each of the carbon atoms are mutually shared, producing two two-electron bonds, an arrangement which is called a double bond. Each carbon in ethene is attached to three other atoms as:. Further, in ethyne acetylene , C2H2, three electrons from each carbon atom are mutually shared, producing three two-electron bonds, called a triple bond, wherein each carbon atom is attached to two other atoms:. Triple bonds are stronger than the equivalent single bonds or double bonds, having a bond order of three. The most common triple bond, that between two carbon atoms, can be found is in alkynes.
The triple bond in ethyne is made up of
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to o , o , or o. According to Valence Shell Electron Pair Repulsion VSEPR theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Carbon is a perfect example showing the value of hybrid orbitals. Carbon's ground state configuration is:. According to Valence Bond Theory , carbon should form two covalent bonds, resulting in a CH 2 , because it has two unpaired electrons in its electronic configuration. Therefore, this does not explain how CH 4 can exist. To form four bonds the configuration of carbon must have four unpaired electrons. One way CH 4 can be explained is, the 2s and the 3 2p orbitals combine to make four, equal energy sp 3 hybrid orbitals. That would give us the following configuration:.
Canned meaning in telugu
Acetylene is a colourless, unpleasant-smelling gas of bp Each carbon atom has two hybrid sp-orbitals, one of which overlaps to form a sp-sp sigma bond with the corresponding one from the other carbon atom. Coordinate linkage is formed Your result is as below. A triple bond is formed between two carbon atoms when three pairs of electrons are shared between them. Get subscription. We will try to answer to the above questions and others through this article. Consider, for example, the structure of ethyne another common name is acetylene , the simplest alkyne. The number of valence electrons in carbon atom is For the bond to form well, there has to be a proper geometrical relationship between the unhybridized p orbitals: they must be in the same plane. Basic First Aid Tips for Kids.
Ethyne, C 2 H 2 , has a triple bond between the two carbon atoms. In the diagram each line represents one pair of shared electrons.
Resonance structure of a molecule cannot have It should also be noted that the carbon-hydrogen bond length in the ethyne molecule is approximately picometres, whereas the carbon-carbon bond length in the molecule is approximately Why are triple bonds the most powerful? Hybrid orbitals are written as spx, where s and p tell the orbitals used for the mixing process, and the value of the superscript x ranges from 1 to 3, depending on how many p orbitals are required in the observed bonding. The formula clearly indicates that it is a hydrocarbon because there are no elements other than carbon and hydrogen in the molecular structure. Coordinate linkage is formed Each carbon atom uses only one of its three p-orbitals. No Yes. The chemical equation for this reaction of calcium carbide and water is shown below. If molecule MX3 has zero dipole moment, the sigma bonding orbitals use Was this answer helpful? This compound is a hydrocarbon because its entire chemical composition consists of only hydrogen and carbon atoms. The number of valence electrons in carbon atom is. Which is the weakest among the following types of bonds.

0 thoughts on “The triple bond in ethyne is made up of”