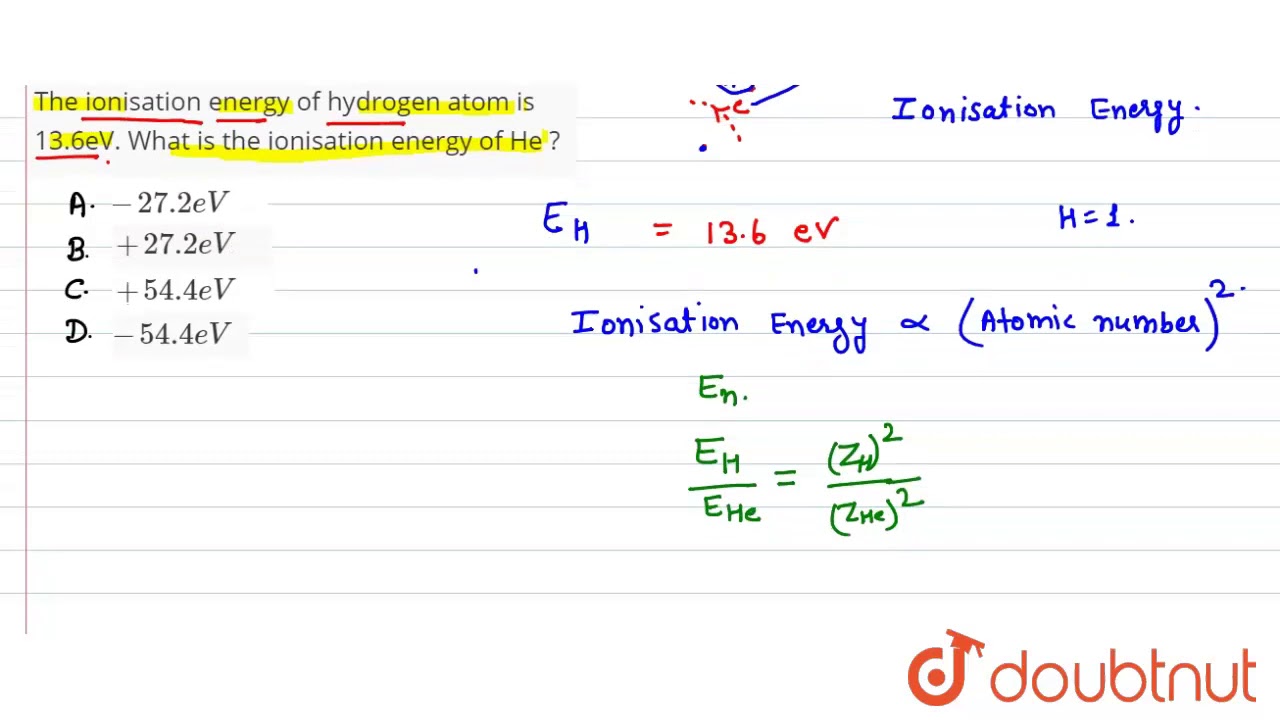
The ionization energy of hydrogen atom is 13.6
Get Started. SSC Exams. Banking Exams.
Ionization potential of hydrogen atom is Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy The spectral lines emitted by hydrogen atoms according to Bohr's theory will be. The ionization potential of H-atom is The H-atoms in ground state are excited by mono chromatic radiations of photon energy
The ionization energy of hydrogen atom is 13.6
Isotopes: The elements which have the same atomic number but different mass numbers are called isotopes. Last updated on May 25, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams. Defence Exams. State Govt. Police Exams. Insurance Exams. Nursing Exams. Other Govt.
NHB Assistant Manager. Agricultural Field Officer - Scale I. MH SET.
The ionisation potential of hydrogen atom is The ionization potential of hydrogen atom is When an electron in the hydrogen atom in ground state absorb a photon of energy If ioinsation potential of hydrogen atom is The ionization energy of hydrogen atom is Hydrogen atoms in the ground state are excited by electromagnetic radiation of energy How many spectral lines will be emitted by the hydrogen atoms.
Last time, we discussed electromagnetic waves and how light is quantized as photons. We related the energy of a photon to its frequency and wavelength using the Planck-Einstein relation:. Electron volts are also a unit of energy, but they are much smaller than a joule. We can convert between joules and electron volts using. Either one works - double checking how your units work out is a great way to check your answer. Planck's constant is.
The ionization energy of hydrogen atom is 13.6
The energies of electrons in molecular orbitals can be observed directly by measuring the ionization energy. This is the energy required to remove an electron, in this case, from a molecule:. Therefore, it requires more energy to remove an electron from the hydrogen molecule than from the hydrogen atom; the electron therefore has a lower energy in the molecule. To pull the atoms apart, the energy of the electron must be increased. Hence, energy is required to break the bond, and the molecule is bound. A bond is formed when the energy of the electrons in the molecule is lower than the energy of the electrons in the separated atoms.
Resin molds india
TS TET. Allahabad High Court Group C. Gujarat High Court Assistant. Bank Note Press Dewas. ICAR Assistant. Assam Forest Protection Constable. Nursing Exams. Gujarat High Court Peon. OSSC Accountant. Cochin Shipyard Executive Trainee. West Bengal Group C.
In physics and chemistry , ionization energy IE is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom , positive ion , or molecule. Roughly speaking, the closer the outermost electrons are to the nucleus of the atom , the higher the atom's ionization energy.
DRDO Fireman. What is the ratio of total kinetic energies in laboratory system TL and centre of mass system TC in the scattering with projectile of mass m1 and target of mass m2? Which of these particles has the shortest De Broglie wavelength. DDA JE. MP Staff Nurse. SBI Clerk. Indian Army Group C. DHS Assam Grade 4. In a radioactive material the activity at time t 1 is R 1 and at a l India Post Mail Guard. How many diggerent spectral lines can one expect when the electron make a downward transition A 1. Tripura TET.

It is necessary to try all
The question is removed