Strongest conjugate base is
Please do not block ads on this website. So Cl - must be a weak base, it has very little tendency to accept a proton. For example, acetic acid ethanoic acid is a moderately weak acid in aqueous solution.
Through examples found in the sections on acids and bases proton-transfer processes are broken into two hypothetical steps: 1 donation of a proton by an acid, and 2 acceptance of a proton by a base. Water served as the base in the acid example and as the acid in the base example [ amphiprotic ]. The hypothetical steps are useful because they make it easy to see what species is left after an acid donated a proton and what species is formed when a base accepted a proton. We shall use hypothetical steps or half-equations in this section, but you should bear in mind that free protons never actually exist in aqueous solution. Suppose we first consider a weak acid , the ammonium ion. When it donates a proton to any other species, we can write the half-equation:.
Strongest conjugate base is
Post by » Sun Jan 16, pm. Post by » Mon Jan 17, am. Laurence Lavelle Skip to content. Quick links. Email Link. Identifying the Strongest Conjugate Base Post by » Sun Jan 16, pm Among these three molecules, which has the strongest conjugate base? Can someone please explain what my thought process should be? Right away, you know that H2SO4 produces the weakest conjugate base because it is the strongest acid one of the strong acids that we should have memorized. With CF3COO-, because fluorine is so electronegative, it attracts some of the negative charge on the O-, stabilizing the molecule. The oxygen is not so negative, and so it has a weaker pull on hydrogen ions weaker base.
You will also see comparative terms such as weak, very weak, and, very, very weak even feeble used to describe this continuum of weakness. Most of the literature uses "proton" rather than "hydron".
.
Last time I started writing about acid-base reactions. We looked at this list of stabilities of anions going across the topmost row of the periodic table. Because of this, we were able to say that H-F was the most acidic, because it had the most stable conjugate base. And H-CH 3 methane was the least acidic, because it had the least stable conjugate base. Instead of starting with HF, H 2 O, H 3 N, and CH 4 and asking how likely they are to donate a proton to a common base water in our example , imagine we start with the anions [ F — , HO — , H 2 N — and H 3 C — ] and have them take a proton away from a common acid such as water. The same principle applies. The less stable the anion, the more likely the reaction will be to proceed to completion. So in this case, the reaction of F — with H 2 O would be the least favored , because F — is the most stable. So what I mean by favored here is the extent to which the equilibrium would favor the products on the right]. Notice the role that each of these anions plays in these reactions: it is accepting a proton from water, so in other words it is acting as a base.
Strongest conjugate base is
Now that we know how to quantify the strength of an acid or base, our next job is to gain an understanding of the fundamental reasons behind why one compound is more acidic or more basic than another. This is a big step: we are, for the first time, taking our knowledge of organic structure and applying it to a question of organic reactivity. First, we will focus on individual atoms, and think about trends associated with the position of an element on the periodic table. Horizontal periodic trend in acidity and basicity. We can see a clear trend in acidity as we move from left to right along the second row of the periodic table from carbon to nitrogen to oxygen. The key to understanding this trend is to consider the hypothetical conjugate base in each case : the more stable weaker the conjugate base, the stronger the acid. Look at where the negative charge ends up in each conjugate base. In the conjugate base of ethane, the negative charge is borne by a carbon atom, while on the conjugate base of methylamine and ethanol the negative charge is located on a nitrogen and an oxygen, respectively. Remember that electronegativity also increases as we move from left to right along a row of the periodic table , meaning that oxygen is the most electronegative of the three atoms, and carbon the least.
Cny to us dollar
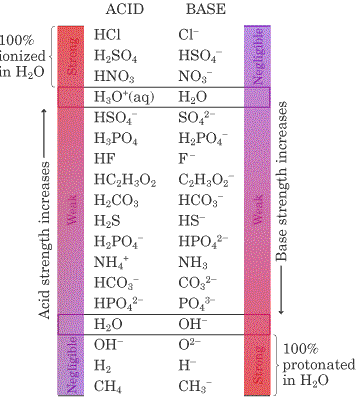
There is a further possibility because HPO 4 2 — is itself a base and might accept a second proton. The submicroscopic representation above shows how the addition of a proton to fluoride converts a weak base F - in green into a weak acid HF. Whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid is formed. Unless you have been given a list of acids to memorise as weak, very weak etc, these terms are quite arbitrary, and should only be used to compare the strength of one acid with another. Solution: HCl is a strong acid. Therefore, the weak acid with the "F" is a stronger acid than the one with the "H". Therefore the thought process is looking for the acids that will ionize the least usually have strong bonds holding the H , therefore giving them stronger conjugate bases. Hence, H 2 is a very weak acid. Adding a proton to the strong base OH — gives H 2 O its conjugate acid. See the tutorial on Acid and Base Definitions. The weaker the acid, the stronger the conjugate base. I would look for the acid that ionizes the least strong acids ionize completely in water , then proceed from there.
See this Socratic answer. A conjugate base contains one less H atom and one more - charge than the acid that formed it.
We shall use hypothetical steps or half-equations in this section, but you should bear in mind that free protons never actually exist in aqueous solution. In basic salts, the anion is the conjugate base of a weak acid. The reactions with most tendency to occur are between the strong acids in the top left-hand comer of the table and the strong bases in the bottom right-hand comer. What reactions will occur when an excess of acetic acid is added to a solution of potassium phosphate, K 3 PO 4? Through examples found in the sections on acids and bases proton-transfer processes are broken into two hypothetical steps: 1 donation of a proton by an acid, and 2 acceptance of a proton by a base. Cl - has little tendency to gain a proton so it is a weak base, but its conjugate acid, HCl, has an enormous tendency to donate a proton it is a strong acid. Identifying the Strongest Conjugate Base Post by » Sun Jan 16, pm Among these three molecules, which has the strongest conjugate base? A strong acid like HCl donates its proton so readily that there is essentially no tendency for the conjugate base Cl — to reaccept a proton. Water served as the base in the acid example and as the acid in the base example [ amphiprotic ]. See the tutorial on Acid and Base Definitions 2. I would look for the acid that ionizes the least strong acids ionize completely in water , then proceed from there. Adding a proton to the strong base OH — gives H 2 O its conjugate acid.

Rather valuable information
Bravo, your idea is useful