Specific heat of hcl
Login to see your most recently viewed materials here. Or if you don't have an account with us yet, then click here to register. Hydrochloric acid is a strong acid used as chemical reagent, pH-regulating, ion exchanger regenerating, pickling or cleaning agent. To see MatWeb's complete data sheet for this material including material property data, metal httyd memes, material suppliers, etcplease click the button below.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table.
Specific heat of hcl
.
Absolute intensities cm-2atm-1 of the band: Benedict, Herman, et al. Boursey, Boursey, E. Part I.
.
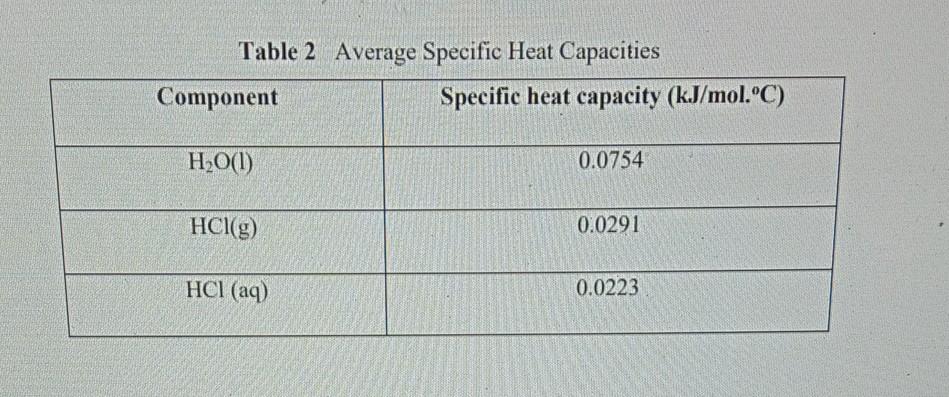
Water has a high capacity for absorbing heat. In a car radiator, it serves to keep the engine cooler than it would otherwise run. As the water circulates through the engine, it absorbs heat from the engine block. When it passes through the radiator, the cooling fan and the exposure to the outside environment allow the water to cool somewhat before it makes another passage through the engine. The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled. The heat that is either absorbed or released is measured in joules. The mass is measured in grams. Calculate the specific heat of cadmium. The specific heat equation can be rearranged to solve for the specific heat.
Specific heat of hcl
Hydrochloric acid , also known as muriatic acid or spirits of salt , is an aqueous solution of hydrogen chloride HCl. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Because it was produced from rock salt according to the methods of Johann Rudolph Glauber , hydrochloric acid was historically called by European alchemists spirits of salt or acidum salis salt acid. Gaseous HCl was called marine acid air. The name muriatic acid has the same origin muriatic means "pertaining to brine or salt", hence muriate means hydrochloride , and this name is still sometimes used. In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi c.
Real pictures of titan moon
Upschulte, Evans, et al. A general reaction search form is also available. Rodova, Levanova, et al. Most recent ab initio values of the ground state molecular constants Meyer and Rosmus, ; charge distribution Cade, Bader, et al. Rank, Birtley, et al. To see MatWeb's complete data sheet for this material including material property data, metal compositions, material suppliers, etc , please click the button below. Recently Viewed Materials most recent at top Login to see your most recently viewed materials here. Users requiring more precise data for scientific or engineering calculations can click on the property value to see the original value as well as raw conversions to equivalent units. Dias, Salema, et al. Tokuhiro, Tokuhiro, T. Rich and Welsh, Rich, N. Kaiser, Kaiser, E. MatWeb is intended for personal, non-commercial use. Moselhy and Pritchard, Moselhy, G. Part 5.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
Mass Spectrom. Rosenberg, Lightman, et al. Transfer , , 12, A: Gen. Press here to zoom. From the photoelectron spectrum Frost, McDowell, et al. Arnett and Pienta, Arnett, E. Wallace, director. Levanova, Treger, et al. Radiative Transfer , , 2, Gebbie and Stone, Gebbie, H. Ion Phys. Inherent substituent parameters from gas phase acidities , J. Very extended progression in absorption, not yet analyzed in detail.

Very curiously :)
Sometimes there are things and is worse
This rather valuable opinion