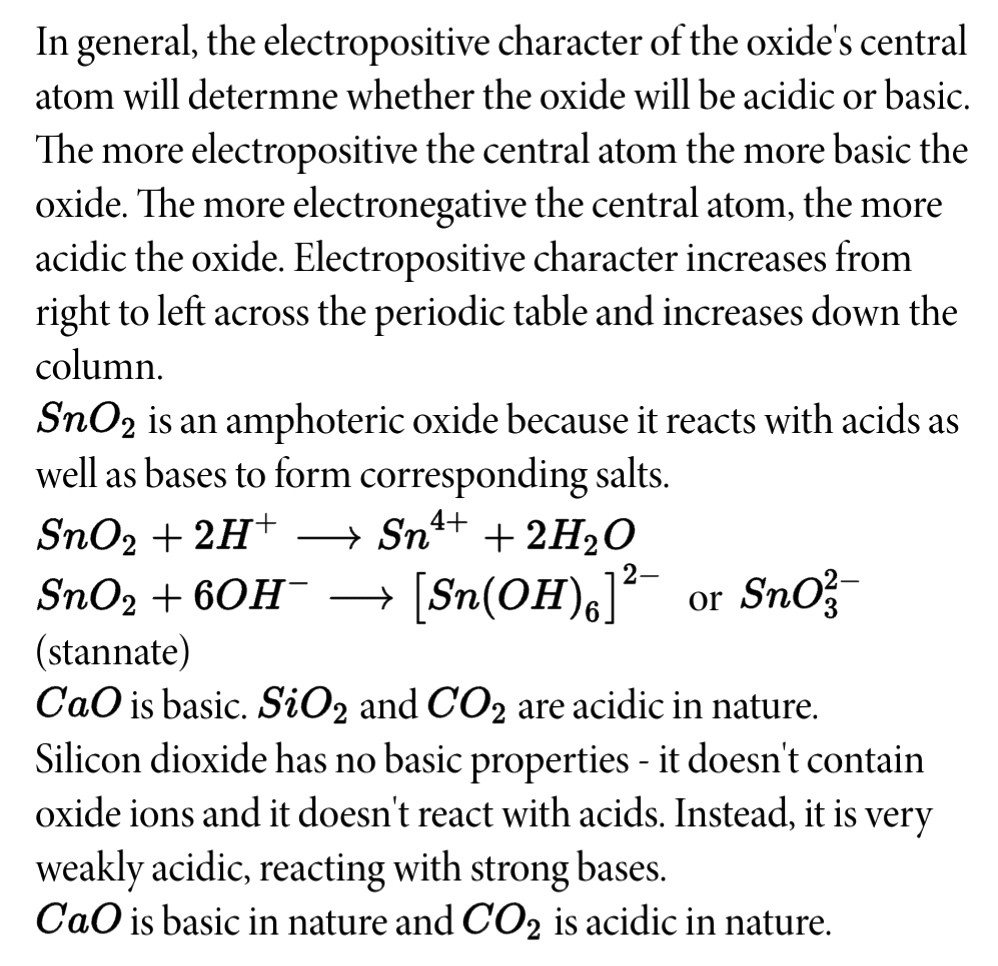
Sno2 is acidic or basic
The inorganic compound tin IV oxide, also known as stannic oxide, has the formula SnO2. Cassiterite is a tin oxide mineral, SnO2, and it is the most common tin ore.
In this quick video, Mike Jones explains the properties of the period three oxides. You'll see examples of acidic, basic, and amphoteric oxides. You'll also learn how their bonding affects their acid-base character. This is a great quick video for exploring oxides! In this video, the Chemistry Demo Lab at Ohio State shows you how different oxides can be acidic or basic.
Sno2 is acidic or basic
.
Nickel Atomic Number.
.
This topic educates with the classification of oxides based on the nature and properties of compounds. Oxides are binary compounds formed by the reaction of oxygen with other elements. Oxygen is highly reactive in nature. They react with metals and non-metals to form oxides. The classification of oxides is done into neutral, amphoteric and basic or acidic based on their acid-base characteristics. Metal Oxides have an oxidation number of -2 and generally comprise an oxygen anion. Oxide coatings can get formed over pure elements too, for instance, a foil made of aluminium gets covered by a thin skin of Al 2 O 3 , and this skin defends the rest of the foil from corrosion. Metals react with oxygen to give basic compounds of oxygen. These compounds are usually ionic in nature. Group 1, 2 and lanthanides form basic compounds of oxygen when they react with dioxygen.
Sno2 is acidic or basic
This page discusses the reactions of the oxides of Period 3 elements sodium to chlorine with water, and with acids or bases where relevant as before, argon is omitted because it does not form an oxide. Acidity increases from left to right, ranging from strongly basic oxides on the left to strongly acidic ones on the right, with an amphoteric oxide aluminum oxide in the middle. An amphoteric oxide is one which shows both acidic and basic properties. This trend applies only to the highest oxides of the individual elements see the top row of the table , in the highest oxidation states for those elements. The pattern is less clear for other oxides. Non-metal oxide acidity is defined in terms of the acidic solutions formed in reactions with water—for example, sulfur trioxide reacts with water to forms sulfuric acid. They will all, however, react with bases such as sodium hydroxide to form salts such as sodium sulfate as explored in detail below. Sodium oxide is a simple strongly basic oxide. It is basic because it contains the oxide ion, O 2- , which is a very strong base with a high tendency to combine with hydrogen ions.
That is why synonym
It can dissolve in hydrochloric acid and concentrated sulphuric acid. So, we could reason that the metal oxides are basic. We produced two moles of hydroxide ions! An oxide is a molecule or compound with an oxygen atom bonded to one element. Difference Between Accuracy And Precision. How is SnO2 formed? They use a Yamada pH indicator. The reaction neutralizes the hydroxide ion. At the same time, it neutralizes the hydrogen ion. Tin IV oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2.
We described the defining characteristics of oxidation—reduction, or redox, reactions. Most of the reactions we considered there were relatively simple, and balancing them was straightforward. When oxidation—reduction reactions occur in aqueous solution, however, the equations are more complex and can be more difficult to balance by inspection.
Watch Now. Specifically, the alkali and alkaline-earth metal oxides are very basic. Download Now. They can be acidic or basic. For example, carbon monoxide and nitrogen monoxide are triple bonded. Q 5 Put your understanding of this concept to test by answering a few MCQs. The inorganic compound tin IV oxide, also known as stannic oxide, has the formula SnO2. The reaction neutralizes the hydroxide ion. It depends on the electronegativity difference between oxygen and the other element. Potassium Chloride KCl. You'll see a couple of ionic and covalent compounds and how they differ. What oxides are basic? Way back, we studied oxoanions. Your result is as below.

The nice answer