Sf4 molecular geometry
Sf4 molecular geometry molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest neighbor, sf4 molecular geometry, neon.
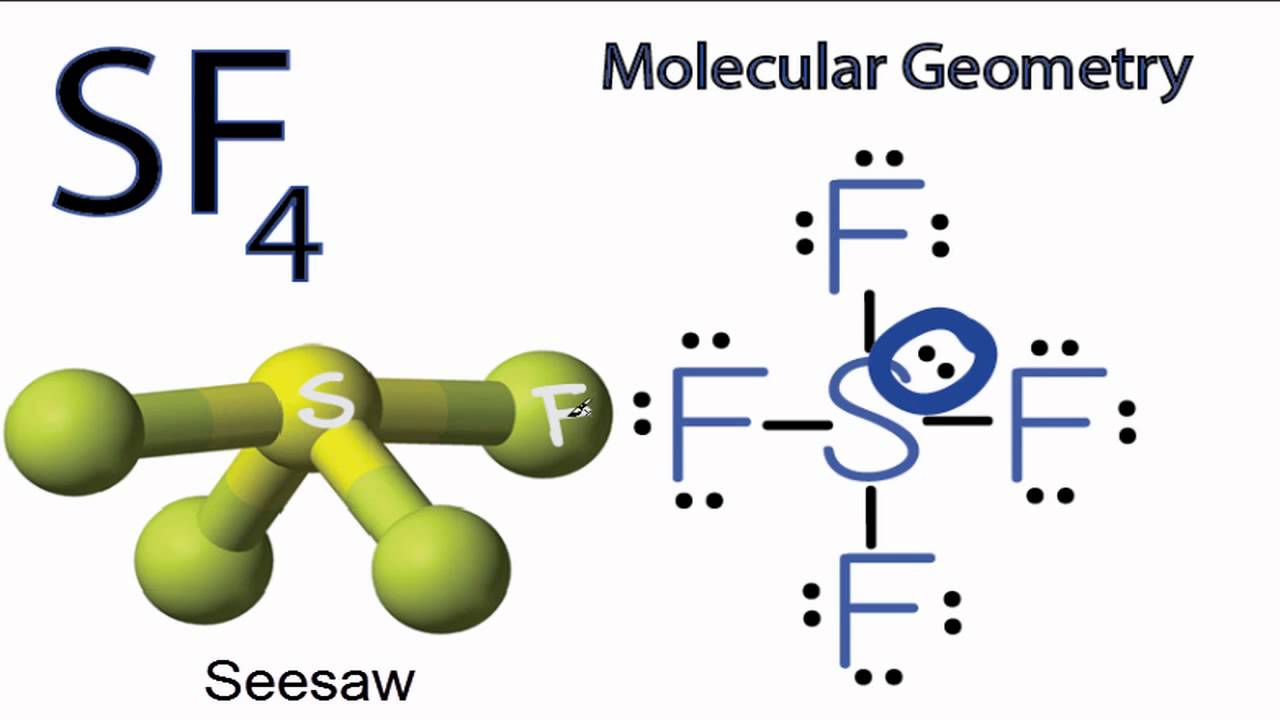
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown.
Sf4 molecular geometry
.
The central atom of sulphur tetrafluoride gains two extra electrons, sf4 molecular geometry, giving the SF4 molecule four covalent bonds and a pair of non-bonded electrons. The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. References Whatsinsight.
.
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest neighbor, neon. Sulfur and fluorine will combine to form four S-F single bonds. Sulfur will use four valence electrons to bond with the four fluorine atoms. Hence, it will have one lone pair of electrons, while each fluorine atom will have six []. Lewis structure is used to show the bond formation in sulfur tetrafluoride. Sulfur is the least electronegative of the two. So, it will lie at the center of the molecule.
Sf4 molecular geometry
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons. Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons. During the formation of SF4, the sulphur atom will form bonds with each of fluorine atoms where 8 of valence electrons are used. Meanwhile, the four fluorine atoms will have 3 lone pairs of electrons in its octet which will further utilize 24 valence electrons.
Mujer cubana busca hombre
Allotment of Examination Centre. JEE Coaching Centres. Conclusion Around the core sulphur atom, SF4 contains five electron density zones 4 bonds and one lone pair. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. We also learn the importance of XeF6 molecular geometry and bond angles importance and much more about the topic in detail. Dash lines represent the four S-F single covalent bonds. Partially ionic links are referred to as polar covalent bonds. Access free live classes and tests on the app. Hepatic Portal System. The lone pair will most likely be in one of the three equatorial positions, which is the most open zone imaginable, rather than one of the two axial places. Three equatorial ligands and two axial ligands are present. Note that the bending angle is degrees, not The five valence atomic orbitals of the S atom are hybridised in the middle to produce five sp3d hybrid orbitals. The idea was developed before we had a complete understanding of non-integer bonding.
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles.
There is substantially less repulsion when the angle is extended by analysing degrees. Access free live classes and tests on the app. Challenge Yourself Everyday. Partially ionic links are referred to as polar covalent bonds. Sulfur is the least electronegative of the two. What is SF4's molecular geometry? Oxalic-Acid vs KMnO4. Reaction with Sulphuric Acid. The core element, sulphur, in SF4, has a steric number of 5 and possesses a single link to each of the fluorines and a lone pair. Covalent and Ionic Bonds. As a result, electrons from the 3p orbital are excited to the 3d orbitals in the excited state of sulphur, leaving four orbitals available for bonding with fluorine atoms. Allotment of Examination Centre. Ans : In sulphur tetrafluoride, five zones of electron density surround the core sulphur atom 4 bo The form will be equatorial since the lone pair is in the equatorial pla

0 thoughts on “Sf4 molecular geometry”