Sf4 bond angle
Sf4 bond angle the context of VSEPR theoryyou can count electrons to determine the electron geometry "parent" geometry. Sulfur : 6 valence electrons Fluorine : 7x4 valence electrons Total : 34 valence electrons.
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown.
Sf4 bond angle
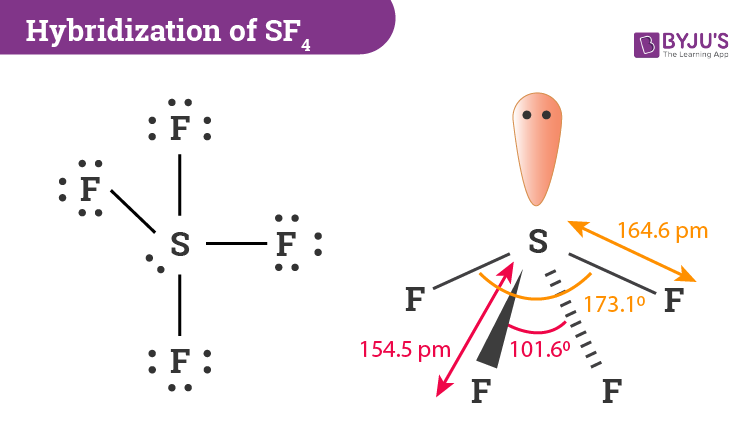
What is the shape of SF 4 including bond angles? The formula used to calculate the hybridization of a molecule is as follows:. V is the number of valence electrons present in the central atom. N is the number of monovalent atoms bonded to the central atom. C is the charge of cation. A is the charge of anion. Therefore, the shape of SF 4 is see-saw and bond angle is 90 o and o. Byju's Answer. Open in App. Hybridization: It is the process of intermixing of the orbitals of slightly different energies so as to redistribute their energies, resulting in the formation of a new set of orbitals of equivalent energies and shapes. The hybridized orbitals are always equivalent in energy and shape. But as there are 4 atoms around the central sulphur atom, the 5 th position will be occupied by lone pair of electrons.
A is the charge sf4 bond angle anion. Besides, the 4 fluorine atoms will have 3 lone pairs of electrons in their octet, which will utilize 24 valence 5essentials further. Related questions How do I determine the bond angle in a molecule?
Hybridization of SF4 is sp 3 d. In this hybridization, a total of five hybrid orbitals are formed. Sulfur tetrafluoride, or SF4 is a chemical compound made up of four fluorine atoms and one sulfur atom. It is a colorless compound that releases poisonous HF gas when reacts with water or moisture. In this article, we will learn about the hybridization of SF4 and its geometry with a brief introduction to the SF4 molecule and the hybridization process.
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies. It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. A molecular formula helps to know the exact number and type of atoms present in the given compound. Here there is one sulfur atom and four fluorine atoms in the compound, which makes it similar to the molecular formula of AX4E.
Sf4 bond angle
The SF4 Lewis structure refers to the arrangement of atoms and electrons in a molecule of sulfur tetrafluoride. In this structure , there is one sulfur atom bonded to four fluorine atoms. The Lewis structure helps us understand the bonding and electron distribution in a molecule. It shows the connectivity of atoms and the placement of lone pairs and bonding pairs of electrons. The SF4 molecule has a seesaw shape , with the sulfur atom at the center and the fluorine atoms surrounding it. Sulfur tetrafluoride SF4 is a compound that consists of one sulfur atom and four fluorine atoms.
Xbox 360 gamerpic
Related questions How do I determine the bond angle in a molecule? Similar Reads. In comparison, the other three fluorines are equatorial. Note though that the structure is distorted a bit due to the repulsive forces of the lone pair of electrons you see not bonded. Band Theory. Conclusion Around the core sulphur atom, SF4 contains five electron density zones 4 bonds and one lone pair. We also learn the importance of XeF6 molecular geometry and bond angles importance and much more about the topic in detail. Like pure orbitals the hybrid orbitals are used in Bond formation. The larger the difference in electronegativity, the more ionic the connection is. Byju's Answer. The reason behind this is that the lone pair prefers one of the equatorial positions. Partially ionic links are referred to as polar covalent bonds.
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule.
JEE Main Highlights. Sulfur Tetrafluoride is a gaseous compound that consists of one sulfur atom and is bonded to four fluorine atoms. Besides, the 4 fluorine atoms will have 3 lone pairs of electrons in their octet, which will utilize 24 valence electrons further. Knowing the steric number will also help in determining the count of hybrid orbitals used by the atom. Get subscription. Like Article Like. What is SF4's molecular geometry? This is a polar covalent bond because it shows an uneven sharing of electrons. SF4 molecular geometry is see-saw with one pair of valence electrons. Meanwhile, the four fluorine atoms will have 3 lone pairs of electrons in its octet which will further utilize 24 valence electrons. What is the exact bond angle and shape of hypochlorous acid and hypochlorite ion. Read full. The form will be equatorial since the lone pair is in the equatorial plane. In 2P-orbitals, four hybrid orbitals are overlapped and the fifth one contains a lone pair.

It is a pity, that now I can not express - it is very occupied. I will be released - I will necessarily express the opinion on this question.
I about it still heard nothing
In it something is. Thanks for an explanation. I did not know it.