Propanone dot structure
Dont't have an account? Register Now.
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. In all cases, the same types of diagrams are used to indicate where electrons and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. An octet rule governs Lewis structures.
Propanone dot structure
.
Post Answer.
.
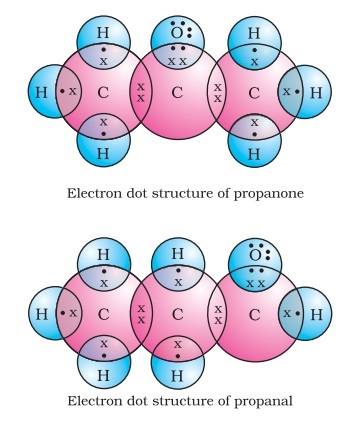
The carbonyl group is ubiquitous in biological compounds. It is found in carbohydrates, fats, proteins, nucleic acids, hormones, and vitamins—organic compounds critical to living systems. In a ketone, two carbon groups are attached to the carbonyl carbon atom. The following general formulas, in which R represents an alkyl group and Ar stands for an aryl group, represent ketones. In an aldehyde, at least one of the attached groups must be a hydrogen atom. The following compounds are aldehydes:. This follows the general rule that in condensed structural formulas H comes after the atom it is attached to usually C, N, or O. The carbon-to-oxygen double bond is not shown but understood to be present. Because they contain the same functional group, aldehydes and ketones share many common properties, but they still differ enough to warrant their classification into two families. The common names of aldehydes are taken from the names of the acids into which the aldehydes can be converted by oxidation.
Propanone dot structure
Propanone is an organic compound having a Ketone group. Today we will discuss how to draw electron dot structure of propanone. Propanone is also called Acetone.
Car oil for peugeot 107
The Lewis structures of hydrogen sulphide H 2 S contain two single bonds surrounding sulphur atoms. Propanone electron dot structure: Posted by Sumit Saini. Management and Business Administration Change. Pharmacy Change. Quick Link BDes M. Phone Number. Online Courses and Certifications Change. Share via. Welcome Back : To keep connected with us please login with your personal information by phone. Computer Application and IT Change. Who do you change sugarcane as black colour turn to white Who they will change the colour of sugarcane black to white. One electron is needed to complete a fluorine octet.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements.
The Lewis structure of H 2 S is based on the number of total valence electrons present in sulphur and hydrogen atoms. Learn Change. In Indian rupees, 1 trillion is equal to how many crores? It is an acetate conjugate acid. Com Colleges in Mumbai Top B. Management and Business Administration Change. Similar Questions for function,why do we write f x? Com Master of Commerce M. Register Now. The Lewis structures of hydrogen sulphide H 2 S contain two single bonds surrounding sulphur atoms.

0 thoughts on “Propanone dot structure”