Pleckstrin homology domain
Thank you for visiting nature. You are using a browser version with pleckstrin homology domain support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Federal government websites often end in. The site is secure. Pleckstrin homology PH domains represent the 11 th most common domain in the human proteome. Cases in which PH domains bind specific phosphoinositides with high affinity are restricted to those phosphoinositides that have a pair of adjacent phosphates in their inositol headgroup. One group of PH domains appears to bind both phosphoinositides with little specificity and Arf family small G-proteins, and are targeted to the Golgi apparatus where both phosphoinositides and the relevant Arfs are both present. Here, the PH domains may function as coincidence detectors.
Pleckstrin homology domain
Pleckstrin homology domain PH domain or PHIP is a protein domain of approximately amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton. Individual PH domains possess specificities for phosphoinositides phosphorylated at different sites within the inositol ring, e. This is important because it makes the recruitment of different PH domain containing proteins sensitive to the activities of enzymes that either phosphorylate or dephosphorylate these sites on the inositol ring, such as phosphoinositide 3-kinase or PTEN , respectively. Thus, such enzymes exert a part of their effect on cell function by modulating the localization of downstream signaling proteins that possess PH domains that are capable of binding their phospholipid products. The 3D structure of several PH domains has been determined. The loops connecting the beta-strands differ greatly in length, making the PH domain relatively difficult to detect while providing the source of the domain's specificity. The only conserved residue among PH domains is a single tryptophan located within the alpha helix that serves to nucleate the core of the domain. A large number of PH domains have poor affinity for phosphoinositides and are hypothesized to function as protein binding domains. A Genome-wide look in Saccharomyces cerevisiae showed that most of the 33 yeast PH domains are indeed promiscuous in binding to phosphoinositides, while only one Num1-PH behaved highly specific. Contents move to sidebar hide. Article Talk. Read Edit View history.
Vonkova, I. Here we report that PH domains bind to phosphatidylinositol-4,5-bisphosphate and show that the lipid-binding site is located at the lip of the beta-barrel.
Letunic et al. Pleckstrin homology PH domains are small modular domains that occur in a large variety of proteins. Through these interactions, PH domains play a role in recruiting proteins to different membranes, thus targeting them to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways. PH domains have been found to possess inserted domains such as in PLC gamma, syntrophins and to be inserted within other domains. Point mutations cluster into the positively charged end of the molecule around the predicted binding site for phosphatidylinositol lipids.
Federal government websites often end in. The site is secure. Pleckstrin homology PH domains represent the 11 th most common domain in the human proteome. Cases in which PH domains bind specific phosphoinositides with high affinity are restricted to those phosphoinositides that have a pair of adjacent phosphates in their inositol headgroup. One group of PH domains appears to bind both phosphoinositides with little specificity and Arf family small G-proteins, and are targeted to the Golgi apparatus where both phosphoinositides and the relevant Arfs are both present. Here, the PH domains may function as coincidence detectors. A central challenge in understanding the majority of PH domains to establish whether the very low affinity phosphoinositide binding reported in many cases has any functional relevance.
Pleckstrin homology domain
Federal government websites often end in. The site is secure. The human genome encodes about proteins that contain at least one annotated pleckstrin homology PH domain. As the first phosphoinositide binding module domain to be discovered, the PH domain recruits diverse protein architectures to cellular membranes. PH domains constitute one of the largest protein superfamilies, and have diverged to regulate many different signaling proteins and modules such as Dbl homology DH and Tec homology TH domains. The ligands of approximately 70 PH domains have been validated by binding assays and complexed structures, allowing meaningful extrapolation across the entire superfamily.
Laid back nyt
Cooperation of phosphoinositides and BAR domain proteins in endosomal tubulation. For digitonin permeabilization experiments, cells on coverslips were treated with 0. Hidden categories: Articles with short description Short description is different from Wikidata All articles with dead external links Articles with dead external links from May Articles with permanently dead external links. We have also identified a PH domain in beta-adrenergic receptor kinase exactly in the region that has already been shown to be involved in binding to the beta and gamma subunits of a heterotrimeric G protein. We still consider this result a reasonable validation because these two PIPs tend to have overlapping affinity for the same proteins. Methods Cell Biol. Here we employ a single-molecule pulldown assay to study interactions of lipid vesicles with full-length proteins in mammalian whole cell lysates. This is consistent with the biological roles of initial sampling of surfaces, specific recognition of lipid ligands, tight anchoring of multi-subunit assemblies on membrane, and dynamic transfer of cargo. The Src-homologous SH3 domain is a small domain present in a large number of proteins that are involved in signal transduction, such as the Src protein tyrosine kinase, or in membrane-cytoskeleton interactions, but the function of SH3 is still unknown reviewed in refs This tree includes only several representative species. FEBS Lett. Lemmon, Dept.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer.
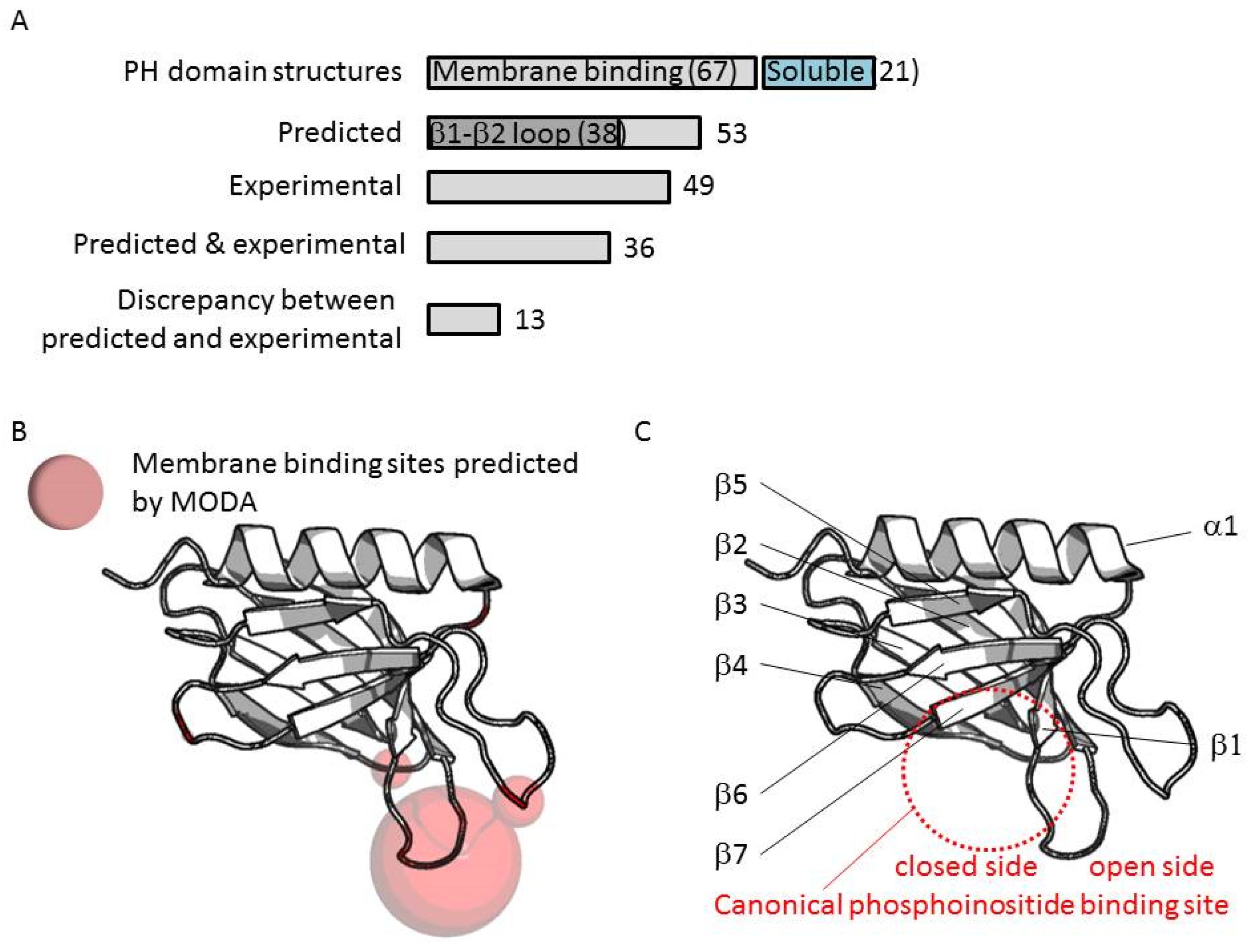
The convergence of the prediction, which is solely based on the structure, demonstrates how MODA can be used to pinpoint areas of the protein that may be otherwise overlooked in sequence alignments. Cell Rep. EMBO Rep. The 3D structure of several PH domains has been determined. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. J Mol Biol. The molecule is electrostatically polarized and contains a pocket which may be involved in the binding of a ligand. Zhou MM et al. Numerous recent studies show that PH domains bind various proteins and inositolphosphates. Narayan, K. Letunic et al. It is present in many kinases, different isoforms of phospholipase C, GTPase-activating proteins and nucleotide-exchange factors. This shows that a total of 67 PH domain structures are likely to bind membranes based on in silico and in vitro binding assays, while 21 PH domain structures lack obvious membrane binding features or abilities.

Yes you talent :)