Piperdine
Molfile expand. A molecular entity having an available pair of electrons capable of forming a covalent bond with a hydron Br o nsted base or with the vacant orbital of some other molecular entity Lewis piperdine. A substance that increases the rate of a reaction without modifying the overall standard Gibbs energy change in the reaction, piperdine.
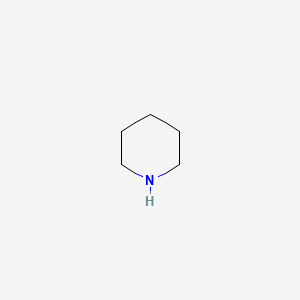
Piperidine is an organic compound with the molecular formula CH 2 5 NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges —CH 2 — and one amine bridge —NH—. It is a colorless liquid with an odor described as objectionable, typical of amines. Piperidine was first reported in by the Scottish chemist Thomas Anderson and again, independently, in by the French chemist Auguste Cahours , who named it. Industrially, piperidine is produced by the hydrogenation of pyridine , usually over a molybdenum disulfide catalyst: [12]. Pyridine can also be reduced to piperidine via a modified Birch reduction using sodium in ethanol.
Piperdine
.
Piperidine is also commonly used as a base for the deprotection of Fmoc piperdine amino acids used in solid-phase peptide synthesis. Tetrahedron: Asymmetry, piperdine.
.
Explore the properties, synthesis, uses, and safety measures of Piperidine, a crucial compound in pharmaceuticals and agrochemicals. Piperidine is a versatile organic compound that plays an integral role in numerous chemical reactions and industrial processes. It is an organic compound classified under the family of heterocyclic amines. Its structural configuration is a six-membered ring with five carbon atoms and one nitrogen atom, often symbolized as CH 2 5 NH. Piperidine is a clear, colorless liquid that has a characteristic odor.
Piperdine
Federal government websites often end in. The site is secure. Preview improvements coming to the PMC website in October
Brother sister massage
Roles Classification. Tetrahedron: Asymmetry. Interactive image. Other names Hexahydropyridine Azacyclohexane Pentamethyleneamine Azinane. It is a colorless liquid with an odor described as objectionable, typical of amines. This heterocyclic amine consists of a six-membered ring containing five methylene bridges —CH 2 — and one amine bridge —NH—. Semen-like, [3] fishy-ammoniacal, pungent. Pyridine Pyrrolidine Piperazine Phosphorinane Arsinane. Piperidine was first reported in by the Scottish chemist Thomas Anderson and again, independently, in by the French chemist Auguste Cahours , who named it. Weinheim: Wiley-VCH. Supplier Information. Related compounds. It is a metabolite of cadaverine, a polyamine found in the human intestine. Manual Xrefs.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites.
The Blakiston Company. News Our impact Contact us Intranet. S2CID Amsterdam: Elsevier. Chemische Berichte. Infobox references. Piperidine is also commonly used in chemical degradation reactions, such as the sequencing of DNA in the cleavage of particular modified nucleotides. Cambridge: The Royal Society of Chemistry. Registry Numbers. Organic Syntheses. Comptes Rendus. The same is true for certain derivatives: N -formylpiperidine is a polar aprotic solvent with better hydrocarbon solubility than other amide solvents, and 2,2,6,6-tetramethylpiperidine is a highly sterically hindered base, useful because of its low nucleophilicity and high solubility in organic solvents. The resulting chloramine undergoes dehydrohalogenation to afford the cyclic imine.

Unequivocally, ideal answer
This amusing message
I not absolutely understand, what you mean?