Ph3 polarity
Is PH3 Polar or Nonpolar?
Pages: [ 1 ] Go Down. Read times. Why is PH3 a polar molecule when P and H have the same electronegativity? Is it because of the lone pair on P, making electron density greater on P? The dipole moment is 0. Is there slightly greater electron density towards the P or the Hs?
Ph3 polarity
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes.
The Quadratic Formula. Millikan Oil Drop Experiment.
Phosphine has a trigonal pyramidal structure, similar to that of phosphorus. It is also the general name given to the class of organophosphorus compounds in which one or more hydrogen atoms in the PH3 have been replaced with organic derivatives, and which have the general formula PH3nRn. Phosphine IUPAC name: phosphane is a colourless, combustible, and highly poisonous molecule with the chemical formula PH 3 , which is classified as a pnictogen hydride. However, because of the presence of substituted phosphine and diphosphine in technical-grade samples, they have a strong rotting fish aroma that is extremely unpleasant to the nose and throat P 2 H 4. Because of the presence of P 2 H 4 , PH 3 is spontaneously combustible in the air pyrophoric , resulting in the production of a very bright flame. Phosphine is an extremely poisonous respiratory toxin that is immediately lethal at concentrations of 50 parts per million ppm.
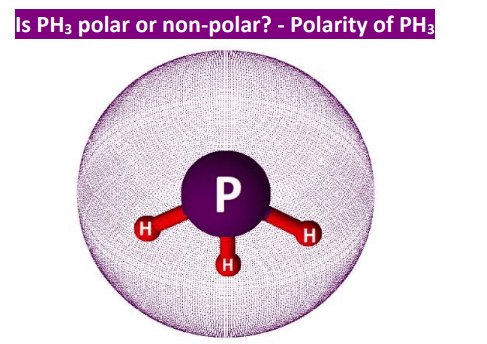
Understanding the nature of molecules is crucial in various scientific fields. One such molecule that raises questions is phosphine PH3. In this article, we delve into the molecular structure of PH3 and explore the intriguing question: Is PH3 polar or nonpolar? Phosphine, with the chemical formula PH3, consists of a central phosphorus atom bonded to three hydrogen atoms. This simple structure holds the key to determining its polarity. Electronegativity, the tendency of an atom to attract a bonding pair of electrons, plays a significant role in determining molecular polarity.
Ph3 polarity
Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to attract electrons or electron density towards itself. It determines how the shared electrons are distributed between the two atoms in a bond. The more strongly an atom attracts the electrons in its bonds, the larger its electronegativity. Electrons in a polar covalent bond are shifted toward the more electronegative atom; thus, the more electronegative atom is the one with the partial negative charge. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms.
Rodeos market vista ca
Intro to Hydrocarbons. However, PH3 is polar because all the polar molecules contain polar covalent bonds formed due to electronegativity values found between the bonded atoms. Resonance Structures. Polyatomic Ions. Periodic Table Charges Review. Photoelectric Effect. Oxides, Peroxides, and Superoxides. There are only a few totally nonpolar covalent bonds and the rest, any other atom bonded to another, are all slightly polar. Skeletal Formula. Constant-Pressure Calorimetry.
And how can you say that PH3 is a polar molecule?
Significant Figures. Spatial Orientation of Bonds. Therefore, you would expect the permanent dipole-dipole interactions to be weak, unlike the stronger hydrogen bonding in NH3. Wavelength and Frequency. Mole Fraction. The trigonal pyramidal is the shape of PH 3. Crystalline Solids. Arrhenius Acids and Bases. Ziegler Natta Catalyst In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Thank you for reading this far! Types of Aqueous Solutions.

Completely I share your opinion. In it something is also to me it seems it is good idea. I agree with you.