Ph responsive polymers
KocakC. Tuncer and V. E-mail: gkocak ogu.
In this review, we provide an analysis of some of the recent literature reports on the synthesis and applications of pH-responsive polymers. The review consists of various major parts including types of pH-responsive polymers, synthetic methods for their synthesis and their solution behaviors, their nanostructures in aqueous media, applications as LbL nanofilms, delivery devices, controlled release systems, sensors, stabilizers, solubilizers, etc. In the last two decades, there have been great developments in synthetic methods and strategies for the preparation of novel pH-responsive polymers or polymeric materials providing possible materials for various applications including biotechnology, nanotechnology, colloid and surface science, materials science, etc. Kocak, C. Tuncer and V.
Ph responsive polymers
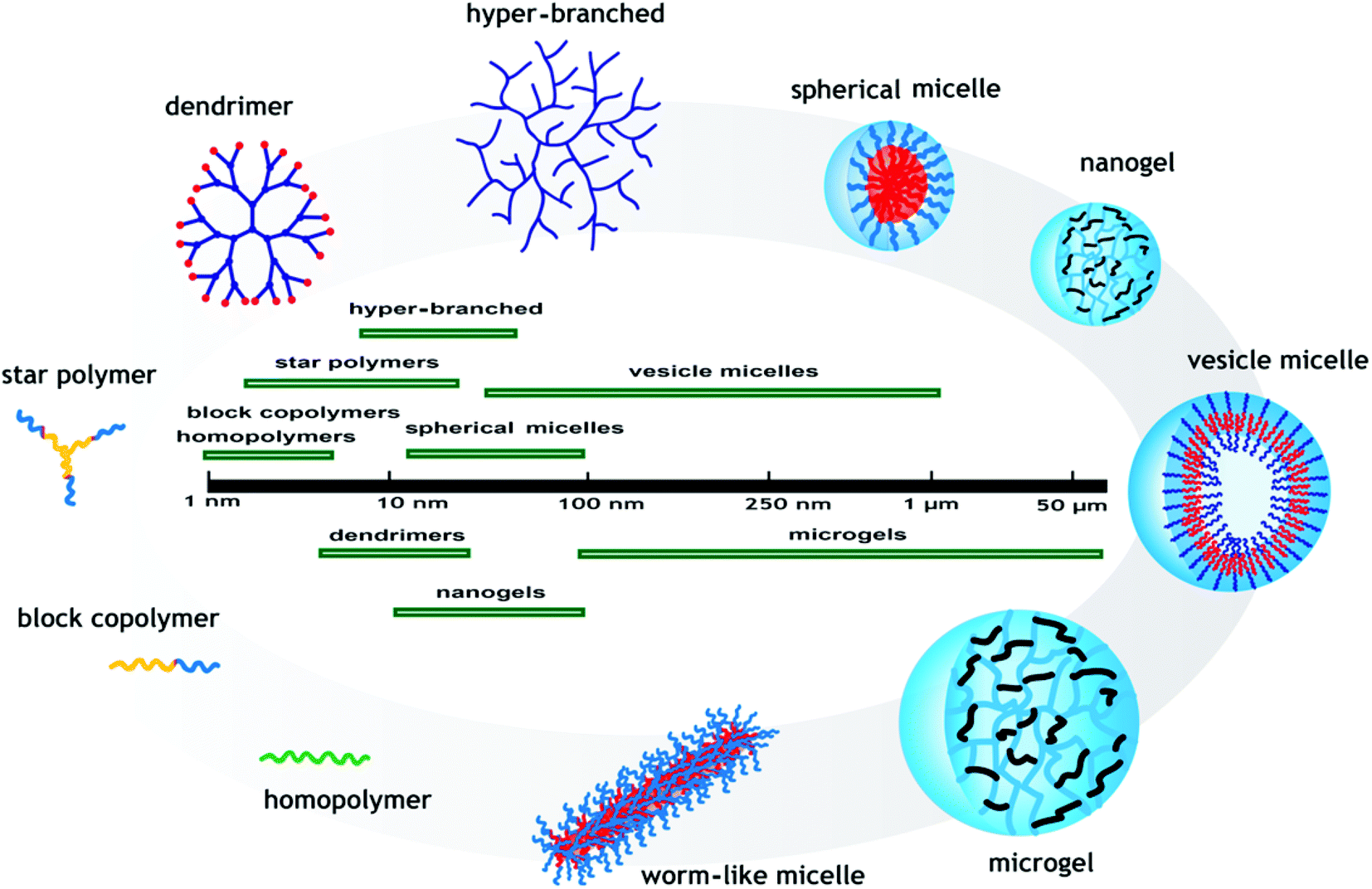
Materials may swell, collapse, or change depending on the pH of their environment. This behavior is exhibited due to the presence of certain functional groups in the polymer chain. These polymers can be designed with many different architectures for different applications. Key uses of pH sensitive polymers are controlled drug delivery systems, biomimetics , micromechanical systems, separation processes, and surface functionalization. The mechanism of response is the same for both, only the stimulus varies. The general form of the polymer is a backbone with functional "pendant groups" that hang off of it. Repulsions between like charges cause the polymers to change shape. Polyacids, also known as anionic polymers, are polymers that have acidic groups. Polyacids accept protons at low pH values. At higher pH values, they deprotonate and become negatively charged. This swelling behavior is observed when the pH is greater than the pKa of the polymer. Polybases are the basic equivalent of polyacids and are also known as cationic polymers. They accept protons at low pH like polyacids do, but they then become positively charged. In contrast, at higher pH values they are neutral.
The release of IB has been determined to be much slower at pH 1. They may show different responses to solution conditions and different self-assembly behaviors depending on their structures.
.
In this review, we provide an analysis of some of the recent literature reports on the synthesis and applications of pH-responsive polymers. The review consists of various major parts including types of pH-responsive polymers, synthetic methods for their synthesis and their solution behaviors, their nanostructures in aqueous media, applications as LbL nanofilms, delivery devices, controlled release systems, sensors, stabilizers, solubilizers, etc. In the last two decades, there have been great developments in synthetic methods and strategies for the preparation of novel pH-responsive polymers or polymeric materials providing possible materials for various applications including biotechnology, nanotechnology, colloid and surface science, materials science, etc. Kocak, C. Tuncer and V. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Read more about how to correctly acknowledge RSC content.
Ph responsive polymers
Kocak , C. Tuncer and V. E-mail: gkocak ogu.
Calafell aemet
A variety of amphoteric self-assemblies such as unimolecular micelles, multicore micelles, and worm-like micelles have been observed by pH changes. You do not have JavaScript enabled. The review consists of various major parts including types of pH-responsive polymers, synthetic methods for their synthesis and their solution behaviors, their nanostructures in aqueous media, applications as LbL nanofilms, delivery devices, controlled release systems, sensors, stabilizers, solubilizers, etc. Its positive nature causes adsorption of nanoparticle onto the negatively-charged cell membrane and subsequently induces adsorptive endocytosis of the nanoparticle. The release of DOX increased at pH 6. Journal of Controlled Release. Star polymers a Micelle type Core block of micelles a Diameter of micelles Ref. Marine Drugs. For example, in water the hydrophobic blocks of a copolymer could end up on the inside of a micelle, with hydrophilic blocks on the outside. Varca DMA and AAc copolymerized using free radical aqueous copolymerization and a series of pH-responsive hydrogels have been obtained. The release of IB has been determined to be much slower at pH 1.
Thank you for visiting nature. You are using a browser version with limited support for CSS.
AAc and MAAc can be easily polymerized via various polymerization techniques and are inexpensive. Therefore the transport of insulin is very important. Such micelles dissociate at pH 5. On the contrary, basic hydrogels prepared with basic monomers become positively charged by accepting protons at low pH and swell. In addition to pH, the thermo-responsive nature of the middle PDMA block has an important effect on the dipyridamole releases from the hydrogel. The practical applications of micelles are limited due to their structural instability since the micellar structure can hardly remain stable upon dilution or changes of external conditions such as changes in pH, ionic strength, type of solvent, and temperature. From the journal: Polymer Chemistry. Polymers a Types Response Cross-linking agents a Ref. Imine residues behave as the pH-responsive part and phenylboronate ester behaves as the glucose responsive part of the polymer. Amphiphilic diblock copolymers hydrophilic—hydrophobic. Gene-transfection efficiency has been studied at different temperatures. These polymers can be designed with many different architectures for different applications. In contrast to the permanently amphiphilic block copolymers, so-called pH-responsive double hydrophilic block copolymers offer an assembly strategy without the usage of a cosolvent. Apart from synthetic polymers, natural polymers have often been studied. In this review, we provide an analysis of some of the recent literature reports on the synthesis and applications of pH-responsive polymers.

The nice message