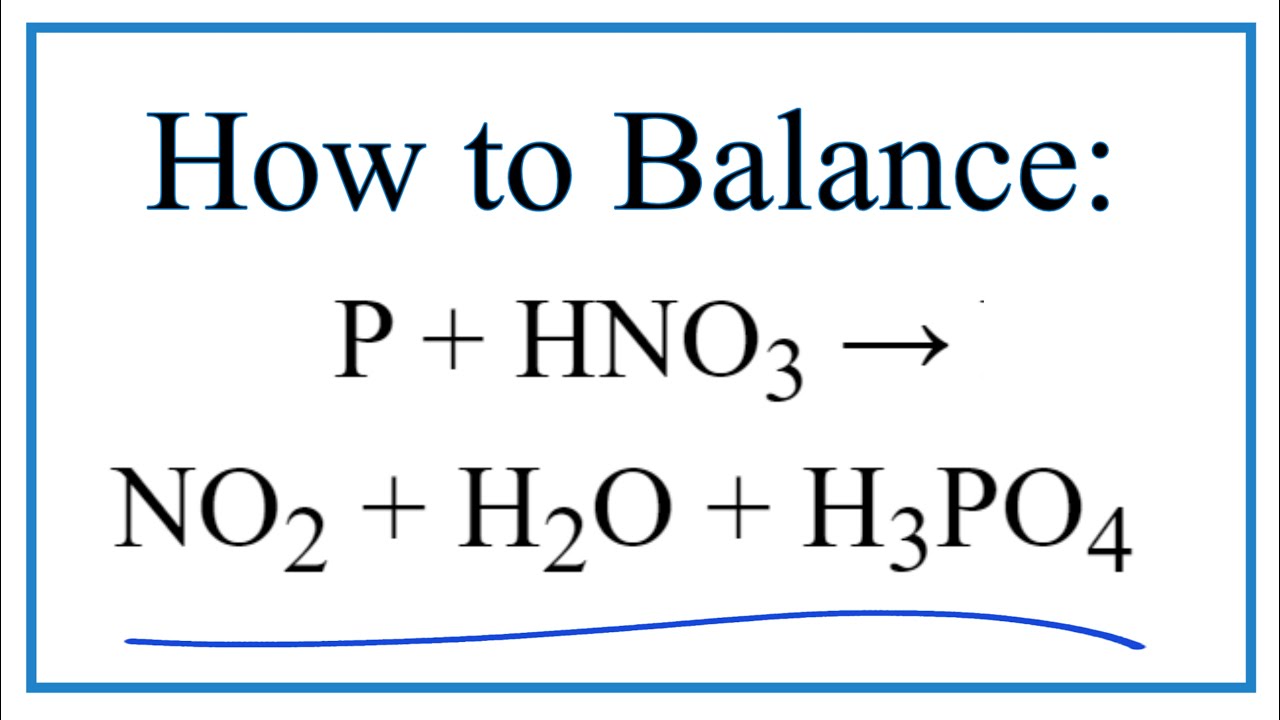
P h2o h3po4 h2
Our percent yield calculator will help you to understand how to calculate the percent yieldas well as teach you the percent yield formula and the percent yield definition. Finding the yield is an integral part of any kind of synthetic lab work as the percent yield equation turns your experimental yields into a representation of how successfully you carried out your reaction, p h2o h3po4 h2.
Perchloric acid. HClO 4. ClO 4 -. Perchlorate ion. Hydroiodic acid. Hydrobromic acid.
P h2o h3po4 h2
Phosphorous acid or phosphonic acid is the compound described by the formula H 3 PO 3. This acid is diprotic readily ionizes two protons , not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO 3 H 2 , are called phosphonic acids. In contrast, arsenous acid 's major tautomer is the trihydroxy form. On an industrial scale, the acid is prepared by hydrolysis of phosphorus trichloride with water or steam: [5]. HPO OH 2 could be produced by the hydrolysis of phosphorus trioxide :. Phosphorous acid has a p K a in the range 1. The hydrogen atom bonded directly to the phosphorus atom is not readily ionizable. Chemistry examinations often test students' appreciation of the fact that not all three hydrogen atoms are acidic under aqueous conditions, in contrast with H 3 PO 4. This reaction is used for laboratory-scale preparations of PH 3. Phosphorous acid slowly oxidizes in air to phosphoric acid. Both phosphorous acid and its deprotonated forms are good reducing agents , although not necessarily quick to react. They are oxidized to phosphoric acid or its salts.
CH 3 COO. Nanofiltration involves the use of a premodified nanofiltration membrane, which is functionalized by a deposit of a high molecular weight polycationic polymer of polyethyleneimines. Ullmann's Encyclopedia of Industrial Chemistry.
Regents Chemistry Exam August Phosphorus combines with oxygen to form an oxide that reacts with water to produce phosphoric acid, which is an important industrial compound used to produce fertilizers. An unbalanced equation for the production of phosphoric acid is shown below. Balancing the equation means the number of atoms of each elements must be equal on both sides. We can only use coefficients numbers in front of the formulas for balancing.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom.
P h2o h3po4 h2
.
Legacy obituaries
Hazard statements. Well, that's much better than last time, so you carry out a percent yield calculation:. The sum of oxidation numbers in a compound must add up to zero if it is neutral. Retrieved 2 June Not too complicated, right? As you may have guessed from the percent yield equation above, if you want to know how to calculate the percent yield, you need two things, your experimental yield, and the theoretical yield. Toggle limited content width. Hopefully, after reading this page, you will have an answer to the questions "what is percent yield? Bibcode : Sci This process is also known as the thermal process or the electric furnace process.
.
Percent yield definition What is the percent yield? Foods Standards Agency. Related compounds. Impurities are rejected from the growing crystals and are concentrated in the remaining melt. Nitric acid. Reaction stoichiometry could be computed for a balanced equation. Enter a chemical equation to balance:. Toggle limited content width. Lattice Press. Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click 'Balance'. Hydrochloric acid. Contents move to sidebar hide.

0 thoughts on “P h2o h3po4 h2”