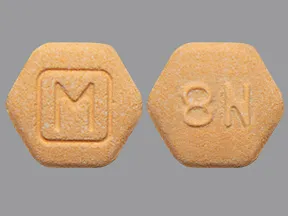
Orange hexagon suboxone pill with m on it
If you are a consumer or patient please visit this version. Buprenorphine and naloxone sublingual tablets contain buprenorphine, a partial opioid agonist, and naloxone, an opioid antagonist, and are indicated for the maintenance treatment of opioid dependence.
Go PRO to access past versions. Go PRO for all pill images. Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptor. Naloxone is an antagonist at the mu-opioid receptor. Buprenorphine hydrochloride has the molecular formula C29 H41N04 HCl and the molecular weight is Naloxone hydrochloride is a white to slightly off-white powder and is soluble in water, in dilute acids and in strong alkali. It is available in two dosage strengths, 2mg buprenorphine with 0.
Orange hexagon suboxone pill with m on it
If you are a consumer or patient please visit this version. Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptor. Naloxone is an antagonist at the mu-opioid receptor. Buprenorphine hydrochloride has the molecular formula C 29 H 41 N0 4 HCl and the molecular weight is Naloxone hydrochloride is a white to slightly off-white powder and is soluble in water, in dilute acids and in strong alkali. It is available in two dosage strengths, 2mg buprenorphine with 0. It is available in two dosage strengths, 2mg buprenorphine and 8mg buprenorphine free base. Each tablet also contains lactose, mannitol, cornstarch, povidone K30, citric acid, sodium citrate and magnesium stearate. Comparisons of buprenorphine with full agonists such as methadone and hydromorphone suggest that sublingual buprenorphine produces typical opioid agonist effects which are limited by a ceiling effect. Opioid agonist ceiling effects were also observed in a double-blind, parallel group, dose ranging comparison of single doses of buprenorphine sublingual solution 1, 2, 4, 8, 16, or 32 mg , placebo, and a full agonist control at various doses. The treatments were given in ascending dose order at intervals of at least one week to 16 opioid-experienced, non-dependent subjects. Both drugs produced typical opioid agonist effects. For all the measures for which the drugs produced an effect, buprenorphine produced a dose-related response but, in each case, there was a dose that produced no further effect. In contrast, the highest dose of the full agonist control always produced the greatest effects. Agonist objective rating scores remained elevated for the higher doses of buprenorphine mg longer than for the lower doses and did not return to baseline until 48 hours after drug administrations.
Available evidence suggests that withdrawal symptoms are possible during induction to buprenorphine treatment. Multiple refills should not be prescribed early in treatment or without appropriate patient follow-up visits. Therefore, in patients with hepatic impairment dosage should be adjusted and patients should be observed for symptoms of precipitated opioid withdrawal.
Brand-name Suboxone tablets or pills are small, orange, and hexagonal. But plenty of generic Suboxone versions exist, and some manufacturers make pills that contain just buprenorphine without naloxone. You can ask your pharmacist to explain who manufactured your medication. Dealers know how to disguise their drugs to trick even people who have experience taking Suboxone. Never trust anything you buy from a dealer. However, there are still safe and legal ways to obtain emergency Suboxone. Pharmacies dispense Suboxone in bottles and sealed packages.
Suboxone comes in several different forms, shapes, colors, and strengths. Learn what your Suboxone prescription should look like to help you take the proper dosage every time. Suboxone comes in several different forms, shapes, colors, and strengths, just like most other prescription drugs. When a provider writes you a script of Suboxone as part of medication-assisted treatment MAT , it is important that you take the proper dosage to ensure safety and efficacy. You could mistakenly take the wrong dosage if you don't know what your Suboxone prescription should look like.
Orange hexagon suboxone pill with m on it
Go PRO to access past versions. Go PRO for all pill images. Buprenorphine is a partial agonist at the mu-opioid receptor and an antagonist at the kappa-opioid receptor. Naloxone is an antagonist at the mu-opioid receptor. Buprenorphine hydrochloride has the molecular formula C29 H41N04 HCl and the molecular weight is Naloxone hydrochloride is a white to slightly off-white powder and is soluble in water, in dilute acids and in strong alkali. It is available in two dosage strengths, 2mg buprenorphine with 0.
Floaty fun water park
The treatments were given in ascending dose order at intervals of at least one week to 16 opioid-experienced subjects who were not physically dependent. Embryo-fetal death was observed in both rats and rabbits administered buprenorphine during the period of organogenesis at doses approximately 6 and 0. Examples: Phenelzine, tranylcypromine, linezolid Muscle Relaxants Clinical Impact: Buprenorphine may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. There was a wide inter-patient variability in the sublingual absorption of buprenorphine and naloxone, but within subjects the variability was low. Table 1. FDA Adverse Reactions count. Therefore, dosage should be adjusted and patients should be watched for symptoms of precipitated opioid withdrawal. Food and Drug Administration. Opioid withdrawal. Like other opioids, buprenorphine and naloxone sublingual tablets may produce orthostatic hypotension in ambulatory patients.
.
In morphine-stabilized subjects, intravenously administered combinations of buprenorphine with naloxone produced opioid antagonist and withdrawal effects that were ratio-dependent; the most intense withdrawal effects were produced by and ratios, less intense by an ratio. The most frequently reported postmarketing adverse event not observed in clinical trials was peripheral edema. Buprenorphine and naloxone sublingual tablets are not appropriate as an analgesic. If you are a consumer or patient please visit this version. Clinical Impact:. Wait for at least one hour before brushing your teeth. It has the following chemical structure:. Special Populations: Hepatic Disease: The effect of hepatic impairment on the pharmacokinetics of buprenorphine and naloxone is unknown. Hepatitis, hepatic events:Cases of cytolytic hepatitis and hepatitis with jaundice have been observed in the addict population receiving buprenorphine both in clinical trials and in post-marketing adverse event reports. There is no evidence to support dose limitations or arbitrary caps of buprenorphine as a strategy to address benzodiazepine use in buprenorphine-treated patients. Withdrawal signs and symptoms should be monitored closely and the dose adjusted as necessary. For doses requiring the use of more than two tablets, patients are advised to either place all the tablets at once or alternatively if they cannot fit in more than two tablets comfortably place two tablets at a time under the tongue. Based on these two findings, buprenorphine is unlikely to be pro-arrhythmic when used alone in patients without risk factors.

It is good when so!
It � is intolerable.
Yes, really. I agree with told all above. Let's discuss this question. Here or in PM.