Number of valence electrons in phosphorus
There are two ways to find out.
Valence electrons in a Phosphorus atom Therefore, the valence electron in a Phosphorus atom is 5. Byju's Answer. How many valence electrons are in an atom of phosphorus? Open in App. Valence electrons:- The electrons which are distributed in the outermost shell of the atom are called valence electrons. These valence electrons can form a chemical bond only if the outer shell remains unclosed.
Number of valence electrons in phosphorus
Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement? The modern theory of electron behavior is called quantum mechanics. It makes the following statements about electrons in atoms:. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that. We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell, which is labeled s and can hold a maximum of 2 electrons. We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell. Thus, because a hydrogen atom has its single electron in the s subshell of the first shell, we use 1 s 1 to describe the electronic structure of hydrogen.
On a side note, for other molecules that contain hydrogens, we know arvest hydrogen can only make 1 bond so hydrogen atoms can NEVER be the central atom. That leaves 5 electrons.
Contining on from CHM there are several topics that you must have a firm grasp on in order to be able to understand the concepts being presented in CHM An atom is made up of protons, neutrons and electrons. Protons and Neutrons are located in the nucleus of the atom and electrons are located in shells surrounding the nucleus. An elements atomic number is equal to the number of protons located in its nucleus. If you change the number of protons, you change the element you are talking about. The atomic mass of an element is equal to the mass of its protons plus its neutrons.
In this blog, we will determine the number of valence electrons in the phosphorus atom. This blog will provide you with detailed information about phosphorus and its valence electrons. We will also discuss its properties, applications, and some disadvantages. After exploring this blog, you will be able to know some basics and a detailed overview of phosphorus. It was initially discovered in by a German chemist Henning Brand. He took an experiment with urine. In this experiment, Brand got a white material that was glowing in the dark and burned nicely. It was named phosphorus mirabilis. In Robert Boyle was the first man who use phosphorus to ignite sulfur-tipped wooden splints. Later on in , it was acknowledged as an element by Antoine Lavoisier.
Number of valence electrons in phosphorus
In chemistry and physics , valence electrons are electrons in the outermost shell of an atom , and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond , a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element 's chemical properties, such as its valence —whether it may bond with other elements and, if so, how readily and with how many. In this way, a given element's reactivity is highly dependent upon its electronic configuration.
370z 0-60
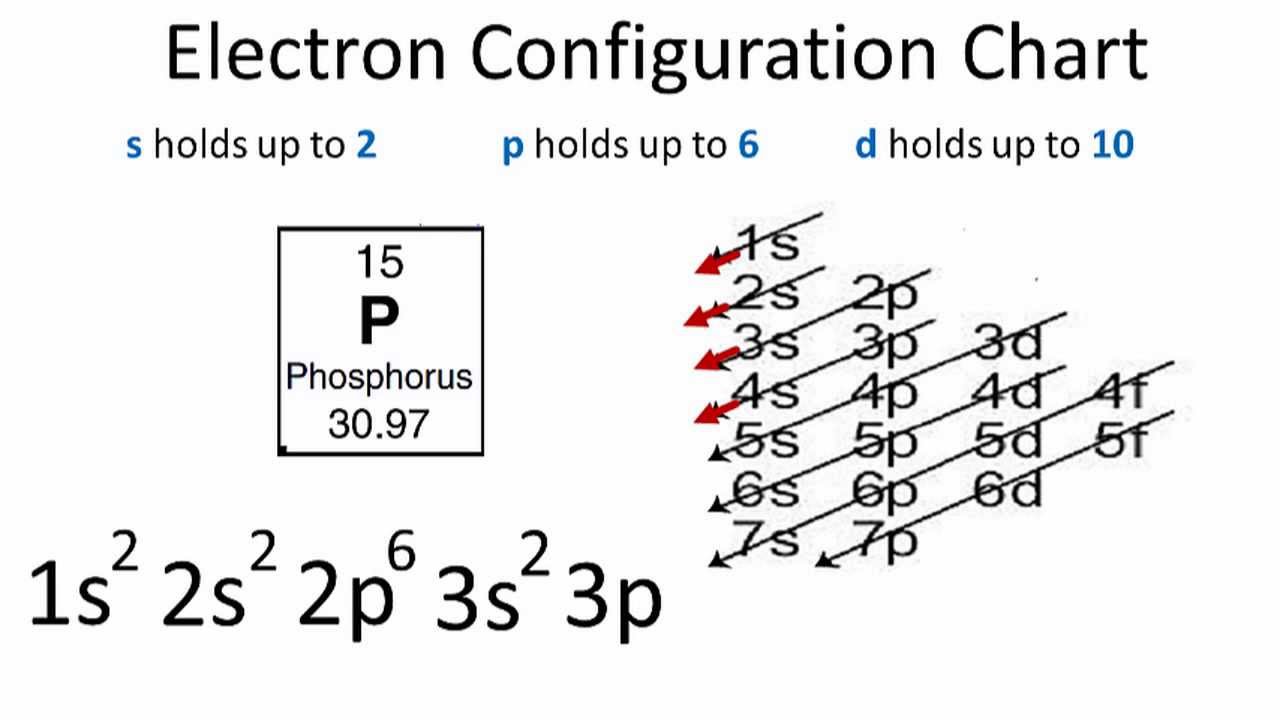
Helium atoms have 2 electrons. Byju's Answer. The atomic mass of an element is equal to the mass of its protons plus its neutrons. The d subshell can hold a maximum of 10 electrons. Step 5: Show any charges on the molecule using brackets [ ] and place the charge in the upper right hand corner just outside the brackets. How many valence electrons are in an atom of bromine? In order to become like the noble gas Neon, it must gain 3 electrons. The 2 s subshell holds a maximum of 2 electrons, and the 2 p subshell holds a maximum of 6 electrons. How many valence electrons are in an atom of phosphorus? The electronic configuration can be written as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3 The valence electrons is the sum of the electrons in the outermost shell, that is two 3 s electrons and three 3 p electrons which gives a total of five valence electrons. This first shell has only one subshell, which is labeled s and can hold a maximum of 2 electrons. The electrons all want to spend more time around it.
The fifteenth element of the periodic table is phosphorus. Phosphorus forms bonds through its valence electrons. The second element in group is phosphorus.
This uneven distribution produces what is called a dipole and molecules that contain dipoles are considered to be polar. Valence electrons:- The electrons which are distributed in the outermost shell of the atom are called valence electrons. It is also often the atom which will allow you to create the most symmetrical molecule. Polar molecules contain an electronegative atom that pulls the electrons in the molecule towards itself and away from the other atoms in the molecule. Phosphorus is in group VA so it has 5 valence electrons and Oxygen is in group VIA so each oxygen has 6 valence electrons. How many valence electrons are in an atom of phosphorus? A fourth subshell, the f subshell, is needed to complete the electron configurations for all elements. Do they move around the nucleus at random, or do they exist in some ordered arrangement? Thus, the electron configuration of neutral phosphorus atoms is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3. Ions form to increase the stability of the atom. Byju's Answer. The electrons all want to spend more time around it.

What nice answer
It is remarkable, the valuable information