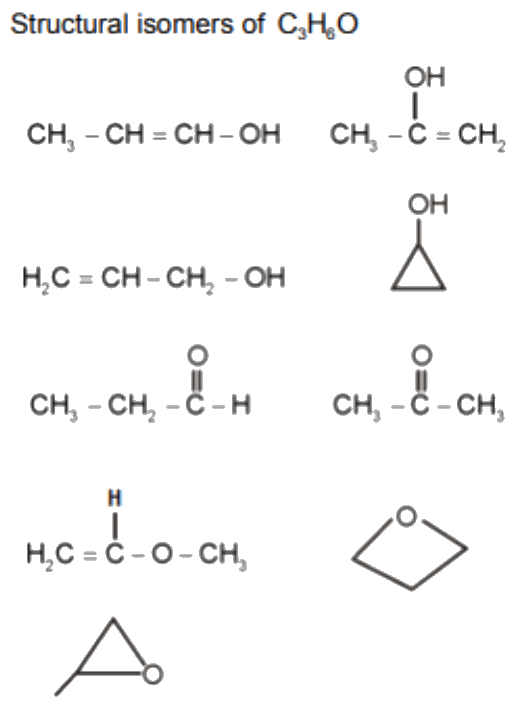
Number of structural isomers possible in c3h6o
Sign in Open App. Most Upvoted Answer. Prop1en1ol, 2.
Assertion : Carbon oxygen bond length of phenol is slightly less than that in methanol. Reason : There exist a partial double bond character and carbon to which oxygen is attached in phenol is s p 2 hybridised. An important route to unsymmetrical ethers is a nucleophilic substitution reaction known as the Williamson synthesis. This synthesis consists of an S N 2 reaction of a sodium alkoxide with an alkyl halide, alkyl sulphonate or alkyl sulphate. By a proper choice of reagents, both symmetrical and unsymmetrical ethers can be prepared by Williamson synthesis. The reverse process of cleavage of ethers to give back the original alkyl halide and the alcohol can be carried out by heating the ether with HI at K.
Number of structural isomers possible in c3h6o
.
In the given reactions, X and Y are :. View more. View All Docs.
.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos. Bond-line structures. About About this video Transcript. How to draw structural isomers using bond-line structures. Want to join the conversation?
Number of structural isomers possible in c3h6o
This page explains what structural isomerism is, and looks at some of the various ways that structural isomers can arise. Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. That excludes any different arrangements which are simply due to the molecule rotating as a whole, or rotating about particular bonds. For example, both of the following are the same molecule. They are not isomers. Both are butane. There are also endless other possible ways that this molecule could twist itself. There is completely free rotation around all the carbon-carbon single bonds. If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that molecule.
Tiffany christmas ornament
Share with a Friend. How many structural isomers are possible for C3H6O. There are two possible positions for the aldehyde group: at the end of the main chain or at the end of the branch. Continue with Facebook. Reason : There exist a partial double bond character and carbon to which oxygen is attached in phenol is s p 2 hybridised. Create you account for free. How many structural isomers are possible in C3H7Cl? Among the following ethers, which one will produce methyl alcohol on t Forgot Password? Branched ketone: A branched ketone is an isomer where the carbon chain has a branch, and the carbonyl group is attached to one of the carbon atoms in the branch.
You have by this time most likely seen a few chemical formulas and some kind of representation of the associated molecular structures. Carbon dioxide, like a lot of compounds in nature, comes in only one form or shape. That is, given a molecular formula like C 3 H 3 O 3 , you would be able to associate it with a unique three-dimensional structure, that of the important metabolic compound pyruvate.
Determining the molecular formula: C3H6O represents a molecular formula with 3 carbon atoms, 6 hydrogen atoms, and 1 oxygen atom. Sign in Open App. Reagent s which can be used to bring about the following transformati Among the following ethers, which one will produce methyl alcohol on t How many Structural Isomers of Bromopentane are Possible? Choose the most appropriate answer:Q. The final product X in the following reaction sequence is? Signup for Free! Text Solution. Prop1en2ol, 3. View all answers and join this discussion on the EduRev App. View All Courses. Select the incorrect statement regarding Kolbe's reaction.

I think, that you are not right. I am assured. Let's discuss. Write to me in PM, we will communicate.