Nnrti full form
Federal government websites often end in.
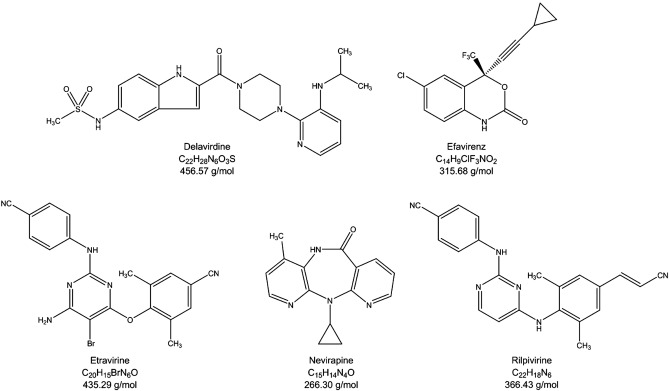
The currently available NNRTIs are nevirapine, delavirdine, and efavirenz; other compounds are under evaluation. NNRTIs are extensively metabolized in the liver through cytochrome P, leading to pharmacokinetic interactions with compounds utilizing the same metabolic pathway, particularly PIs, whose plasma levels are altered in the presence of NNRTIs. NNRTIs are drugs with a low genetic barrier, i. However, due to the rapid emergence of resistant virus to these compounds in case of incomplete viral suppression, NNRTIs should not be added to current failing antiretroviral regimen. The most common side-effect reported with nevirapine and delavirdine is rash. The incidence of rash is rather similar under these two compounds, but severe rash is less frequent with delavirdine. The most common adverse reactions reported with efavirenz are central nervous system complaints such as dizziness.
Nnrti full form
The viral DNA is then integrated into the host chromosomal DNA, which then allows host cellular processes, such as transcription and translation, to reproduce the virus. RTIs block reverse transcriptase's enzymatic function and prevent completion of synthesis of the double-stranded viral DNA, thus preventing HIV from multiplying. A similar process occurs with other types of viruses. Some of the same compounds used as RTIs can also block HBV replication; when used in this way they are referred to as polymerase inhibitors. The antiviral effect of NRTIs and NtRTIs is essentially the same; they are analogues of the naturally occurring deoxynucleotides needed to synthesize the viral DNA and they compete with the natural deoxynucleotides for incorporation into the growing viral DNA chain. NNRTIs block reverse transcriptase by binding directly to the enzyme. NNRTIs are therefore classified as non-competitive inhibitors of reverse transcriptase. This phosphorylation step is carried out by cellular kinase enzymes. NRTIs can induce mitochondrial impairment that leads to a number of adverse events, including symptomatic lactic acidosis. As described above, host cells phosphorylate nucleoside analogs to nucleotide analogs. Taking phosphonate nucleotide analog reverse-transcriptase inhibitors NtARTIs or NtRTIs directly obviates the initial phosphorylation step, but host enzymes must still phosphorylate the phosphonate nucleotide analogue to the phosphonate-diphosphate state for anti-viral activity. These molecules were first synthesized by Antonin Holy at the Czech Academy of Sciences , and commercialized by Gilead.
However, due to the rapid emergence of resistant virus to these compounds in case of incomplete viral suppression, NNRTIs should not be added to current failing antiretroviral regimen.
.
RT is one of the most popular targets in the field of antiretroviral drug development. Discovery and development of NNRTIs began in the late s [2] and in the end of four NNRTI had been approved by regulatory authorities and several others were undergoing clinical development. Acquired immunodeficiency syndrome AIDS is a leading cause of death in the world. In the year over 40 million people were infected worldwide with HIV and the number keeps on growing. The drugs belong to six different classes that act at different targets. The most popular target in the field of antiretroviral drug development is the HIV-1 reverse transcriptase RT enzyme.
Nnrti full form
Federal government websites often end in. The site is secure. This review describes recent clinical data, pharmacokinetics, metabolism, pharmacodynamics, safety and tolerability of commercialized NNRTIs, including the effects of sex, race and age differences on pharmacokinetics and safety. This review presents a wide description of NNRTIs, providing useful information for researchers interested in this field, both in clinical use and in research. Infections with the human immunodeficiency virus HIV are typically treated with drug combinations consisting of at least three different antiretroviral drugs. Many of these regimens have comparable efficacy but vary to some degree in dosing frequency, pill burden, drug interactions and potential side effects. The choice of a regimen for a given individual is based on expected side effects, convenience, comorbidities, interactions with concomitant medications and genotypic drug-resistance testing [ 1 ]. Recent data suggest that virologic failure on first-line regimens mostly occurs due to either pre-existing transmitted drug resistance or suboptimal adherence [ 1 ]. Therefore, genotypic resistance testing and adherence to the treatment are fundamental criteria when selecting the most optimal initial antiretroviral regimen.
2000000 idr to gbp
In other projects. Review Questions Access free multiple choice questions on this topic. Postexposure prophylaxis is attainable through either one of the following regimens for four weeks:. Toggle limited content width. Molecular Cell. In all cases, patents remain in force until beyond Physicians can also work closely with pharmacists to help optimize treatment and give the best medications to patients to achieve optimal therapy; the pharmacist can consult on the best combinations of agents and optimal dosing and administration. PMID Patients should understand the serious adverse effects the drugs that make up their HAART regimen can cause. Improvement in lipoatrophy associated with highly active antiretroviral therapy in human immunodeficiency virus-infected patients switched from stavudine to abacavir or zidovudine: the results of the TARHEEL study.
Federal government websites often end in.
References 1. Download as PDF Printable version. October Russian State Register of Medicines in Russian. Journal of Molecular Biology. Similar articles in PubMed. Critical Care. The majority of the adverse effects are seen in chronic uses situations rather than sudden onset and associated with each subcategory of the drug. The currently available NNRTIs are nevirapine, delavirdine, and efavirenz; other compounds are under evaluation. A review of the toxicity of HIV medications. The primary mechanism of action is through the binding of the NNRTI to the reverse transcriptase and the creation of a hydrophobic pocket proximal to the active site. Their binding results in a conformational change in the reverse transcriptase that distorts the positioning of the residues that bind DNA, inhibiting polymerization. Steroidogenesis inhibitor.

0 thoughts on “Nnrti full form”