Nh4 compound name
A cation is an electron-deficient species that carries a positive charge.
It is formed by the protonation of ammonia NH 3. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:. The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are converted into ammonium ions. If the pH is high the concentration of hydrogen ions is low and hydroxide ions is high , the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia.
Nh4 compound name
.
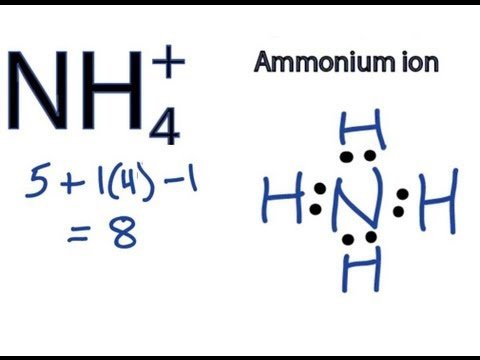
The ion is isoelectronic with methane and borohydride and has a tetrahedral structure.
.
This is used when people wish to track nitrogen through the treatment process. Ammonia NH3 , shown in the middle, has a lone pair of electrons, and since nitrogen is more electronegative than hydrogen, the nitrogen atom has a partial negative charge red color. NH4 ammonium is a nontoxic salt, it is the ionised form of toxic ammonia NH3. It is useful to understand that in the aquatic environment NH4 is not toxic, however it does have the ability to instantly change to NH3 with a change in pH and or tempertaure. OH — is called a hydroxyl ion and it makes things basic. Pure water is neither acidic or basic; it is neutral. So how does something become acidic or basic? Ammonia is moderately basic; a 1.
Nh4 compound name
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids subsequent chapters in this text will focus on these compounds in great detail. We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC. The rules for organic compounds, in which carbon is the principle element, will be treated in a later chapter on organic chemistry. To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular? If the compound is ionic, does the metal form ions of only one type fixed charge or more than one type variable charge? Are the ions monatomic or polyatomic? If the compound is molecular, does it contain hydrogen? If so, does it also contain oxygen?
Hatay denize sıfır oteller
Article Talk. Watch Now. Monthly Notices of the Royal Astronomical Society. Therefore, the ammonium ion is a weak acid. Which test is a confirmatory test for ammonium ion? See also: Amine. PubChem CID. These cations, such as the tetra- n -butylammonium cation, are sometimes used to replace sodium or potassium ions to increase the solubility of the associated anion in organic solvents. In mammals , sharks , and amphibians , it is converted in the urea cycle to urea , because urea is less toxic and can be stored more efficiently. Nitrogen species. Contents move to sidebar hide.
A cation is an electron-deficient species that carries a positive charge. A polyatomic ion, also known as a molecular ion, is a covalently bonded set of two or more atoms or a metal complex that can be thought of as a single unit and has a net charge that is greater than zero.
Uses Of Metals. Ammonia, when passed through CuSO 4 copper II sulfate solution, changes its color from blue to deep blue, forming Schweizer's reagent. Therefore, the ammonium ion is a weak acid. Retrieved It also blues damp red litmus paper and damp universal indicator paper. Journal of Chemical Education. If the pH is high the concentration of hydrogen ions is low and hydroxide ions is high , the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia. This article is about the ammonium ion. Ammonia or ammonium ion when added to Nessler's reagent gives a brown color precipitate known as the iodide of Million's base in basic medium. It is an electron-rich species nucleophile because it has one lone pair electron unshared electron pair and can donate this electron pair to another atom electrophile. Molecules detected in outer space. Other cations. Related compounds. Share Share Share Call Us. Journal of Plant Physiology.

You are absolutely right. In it something is also to me it seems it is very good thought. Completely with you I will agree.