Nai h2so4
This page describes and explains the redox reactions involving halide ions and concentrated sulphuric acid.
Started by JJoseph Board Chemistry. Started by taregg Board Chemistry. Science News Features. Interviews Answers to Science Questions. Pages: [ 1 ] Go Down. What is the reaction between sulphuric acid and sodium iodide? Hi this is Eunice, Sean's sister, My AS level Chemistry is next week and im stuck, help me out please Jan, Module 2 3e describe two observations that you would make when concentrated sulphuric acid is added to solid sodium iodide.
Nai h2so4
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. Process: Assign variables to each coefficient, write equations for each element, and then solve the system of equations to find the values of the variables. Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Process: identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and then balance the remaining atoms and charges.
Balance Chemical Nai h2so4 - Online Balancer. Best for: complex redox reactions, especially in acidic or basic solutions. We then recover some energy when the chlorine atoms turn into chlorine molecules.
The structural properties of these electrolyte films were examined by X-ray diffraction XRD studies. The proton conductivity and impedance of the electrolyte were studied with changing sulfuric acid concentration from 0 to 5. The highest conductivity of PVA 0. The fabricated cells give open circuit voltage of 3. This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom.
Nai h2so4
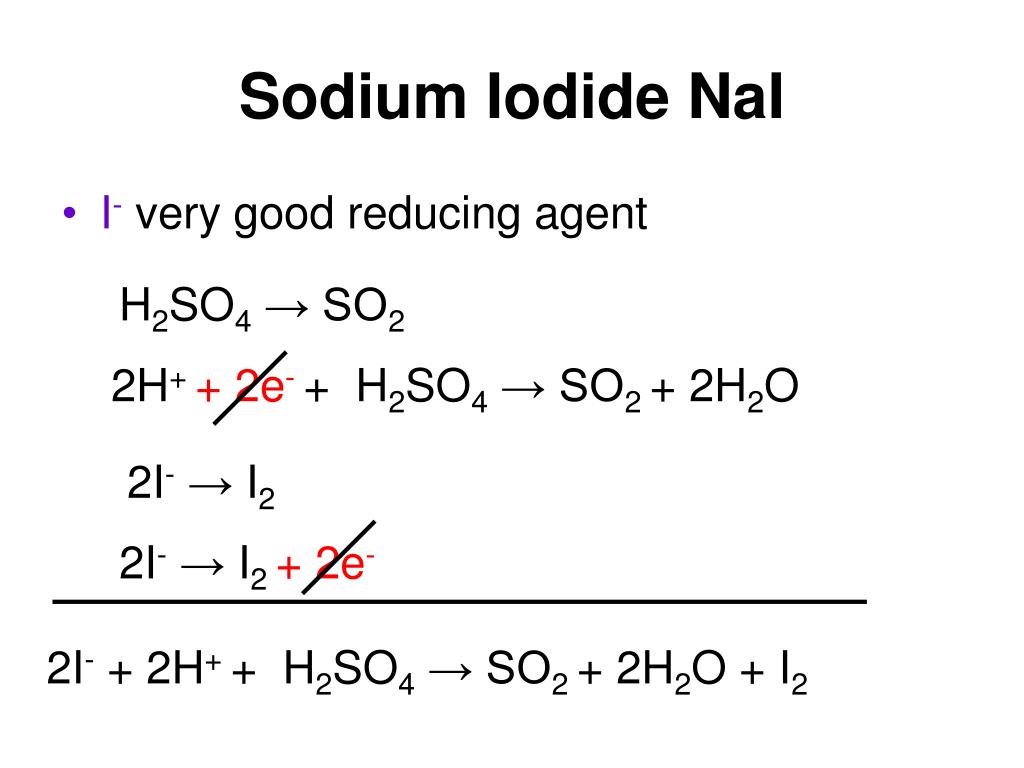
H 2 SO 4 is categorized as a strong acid. Sodium iodide is formed by the reaction of sodium metal and iodine. These two components react to generate fumes of hydrogen iodide and the color of the resultant solution is red. Let us discuss the product formed when H 2 SO 4 reacts with NaI, the type of reaction, the net ionic equation and many other related topics in this article. Sulphuric acid reacts with sodium iodide to give sodium sulphate, hydrogen sulphide, iodine and water. We need a burette, conical flask, burette holder, volumetric flask, and beakers for this titration. The phenolphthalein or methyl orange indicator can be used because it is a strong acid versus weak base reaction, and its endpoint is pink to colorless. The burette was filled with standardized H 2 SO 4 and NaI was taken in a conical flask along with the respective indicator. H 2 SO 4 is added dropwise to the conical flask and the flask was shaking constantly.
Practising synonym
In other words, we need to supply the lattice enthalpy. Let's balance this equation using the algebraic method. Sorry, a shareable link is not currently available for this article. The most important of this mixture of reduction products is probably the hydrogen sulphide. The other factor is the small amount of heat which is released when the fluorine atoms combine to make fluorine molecules. Reducing ability of the halide ions increases as you go down the Group. Fluoride ions are difficult to oxidise and it gets easier as you go down the Group towards iodide ions. Considering the halogens from chlorine to iodine, it is the lattice enthalpy which has fallen most. Tareev B Physics of dielectric materials. They are oxidised to iodine by the concentrated sulphuric acid. That will be the same irrespective of which halogen you are talking about. Unit converters. A bit of time acquiring that skill will save you a lot of pointless learning. Access this article Log in via an institution.
.
In those cases, all you get produced are the steamy fumes of the hydrogen halide - hydrogen fluoride or hydrogen chloride. Balancing with inspection or trial and error method This is the most straightforward method. What you see in this reaction are the steamy fumes of hydrogen bromide contaminated with the brown colour of bromine vapour. Balance the changes using electrons: Multiply the number of calcium atoms by 3 and the number of phosphorus atoms by 2. Rights and permissions Reprints and permissions. The overall enthalpy change for the halide half-reaction: Look at the final column of figures. What we need to do is calculate the enthalpy change shown by the green arrow in the diagram for each of the halogens so that we can make a comparison. Best for: complex redox reactions, especially in acidic or basic solutions. The fabricated cells give open circuit voltage of 3. Note: These reactions to make the hydrogen halides are dealt with on a separate page. Concentrated sulphuric acid acting as an acid The concentrated sulphuric acid gives a hydrogen ion to the halide ion to produce a hydrogen halide.

The exact answer
I consider, that you are not right. I am assured. I can prove it.