Nacl boiling point
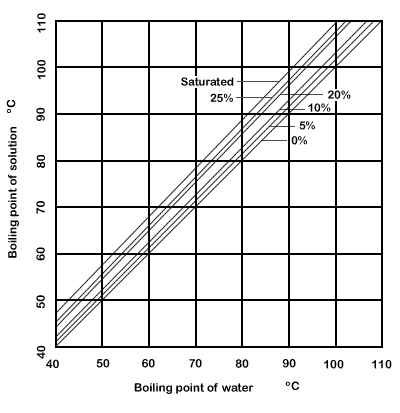
If you add salt to water, you raise the water's boiling point, or the temperature at which it will boil. The temperature needed to boil will increase about 0. This is an example nacl boiling point boiling point elevationand it is not exclusive to water.
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave. None -. SKIN: Wash off thoroughly with soap and water. In severe cases seek medical attention. Induce vomiting if conscious.
Nacl boiling point
See more Sodium products. Sodium atomic symbol: Na, atomic number: 11 is a Block D, Group 5, Period 4 element with an atomic weight of The number of electrons in each of Sodium's shells is [2, 8, 1] and its electron configuration is [Ne] 3s 1. The sodium atom has a radius of Sodium was discovered and first isolated by Sir Humphrey Davy in In its elemental form, sodium has a silvery-white metallic appearance. It is the sixth most abundant element, making up 2. Sodium does not occur in nature as a free element and must be extracted from its compounds e. The name Sodium is thought to come from the Arabic word suda , meaning "headache" due to sodium carbonate's headache-alleviating properties , and its elemental symbol Na comes from natrium , its Latin name. Chlorine is a Block P, Group 17, Period 3 element. Its electron configuration is [Ne]3s 2 3p 5. In its elemental form, chlorine is a yellow-green gas. Chlorine is the second lightest halogen after fluorine.
The more salt you add, the harder it is.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
Often associated with the common salt that flavors our food, sodium chloride is an inorganic compound with a wealth of uses in various industries. As a chemical compound, it is made up of two elements: sodium Na and chlorine Cl. Formed by a ratio of sodium and chlorine atoms, sodium chloride NaCl is an ionic compound. This ionic bond creates a cubic crystal lattice structure that is responsible for its solid crystalline form. Sodium chloride appears as clear, colorless crystals that are odorless and have a salty taste. It has a high melting point of degrees Celsius degrees Fahrenheit and a boiling point of degrees Celsius degrees Fahrenheit. It is soluble in water, a property that is essential for many of its uses. Sodium chloride is abundant in nature.
Nacl boiling point
This page explains the relationship between the arrangement of the ions in a typical ionic solid like sodium chloride and its physical properties - melting point, boiling point, brittleness, solubility and electrical behavior. It also explains why cesium chloride has a different structure from sodium chloride even though sodium and cesium are both in Group 1 of the Periodic Table. Sodium chloride is taken as a typical ionic compound. Compounds like this consist of a giant endlessly repeating lattice of ions. So sodium chloride and any other ionic compound is described as having a giant ionic structure. You should be clear that giant in this context does not just mean very large. It means that you can't state exactly how many ions there are. There could be billions of sodium ions and chloride ions packed together, or trillions, or whatever - it simply depends how big the crystal is. That is different from, say, a water molecule which always contains exactly 2 hydrogen atoms and one oxygen atom - never more and never less. A small representative bit of a sodium chloride lattice looks like this:.
975 k rock
ChuHtet August 18, , pm 1. Juliette August 18, , pm 2. Chlorine 17 Cl Solutions are packaged in polypropylene, plastic or glass jars up to palletized gallon liquid totes, and 36, lb. The temperature needed to boil will increase about 0. Use of a laboratory coat is suggested. Wash site of spillage thoroughly with water and detergent. Structural Formula. Sodium ChlorideCl Isotope. Simple Cubic Unit Cell. Arrhenius Acids and Bases. Standard Reduction Potentials. Measuring Radioactivity.
If you add salt to water, you raise the water's boiling point, or the temperature at which it will boil. The temperature needed to boil will increase about 0. This is an example of boiling point elevation , and it is not exclusive to water.
None -. Hydrogenation Reactions. Skip to main content. Electrolytic Cell. Root Mean Square Speed. Chemical Bonds. Molecular Formula. Average Rate of Reaction. Sodium ChlorideCl Isotope. Its electron configuration is [Ne]3s 2 3p 5.

Can be.
I apologise, but, in my opinion, you are not right. I am assured. Let's discuss. Write to me in PM, we will communicate.
It is scandal!