Moles to moles calculator
We've updated our Privacy Policy to make it clearer how we use your personal data. We use cookies to provide you with a better experience. You can read our Cookie Policy here.
Our molar ratio calculator will help you determine the molar ratio between the different chemicals reacting and the different chemicals produced during the reaction. It can also help you determine the mass or the number of moles of each chemical required to perform the reaction. Equipped with that knowledge, you can find the limiting agent in the reaction or which chemical is available in excess. Are you wondering what is a molar ratio and how to calculate it? Do you what to learn the importance of a molar ratio?
Moles to moles calculator
Want to know how to calculate moles? Need a grams-to-moles calculator or even a moles-to-grams calculator? Well, then, you've come to the right place. With our moles-to-grams converter, you can seamlessly convert between mass, molecular weight, and moles. Chemistry just became that little bit easier! Impress your friends with your astounding ability to find how many moles of a substance you have at a kilogram, ounce, or even tonne scale! Also useful for any serious industrial applications, for all you chemical engineers out there. While we're on the topic of chemistry, we have several other calculators that you might find useful. Why not check out our molarity calculator or our percent yield calculator? A mole is a small, subterranean mammal belonging to the family Talpidae. Just kidding — we're sure you've never heard that joke before.
Let's do a quick example to help explain how to convert from moles to grams or grams to moles. Table of contents What is a mole? Molecular mass also called molecular weight - sum of the atomic weights of all atoms appearing in a given molecular formula.
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation. The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients. However, the equation is balanced as long as the coefficients are in a ratio. For example, this equation is also balanced if we write it as. The ratio of the coefficients is , which reduces to
In the previous section , several relationships were written, including:. These relationships may be used to convert from grams to moles or vice versa; or from moles to atoms, molecules, or formula units or vice versa. In the next section , we will show how these relationships may also be used to count atoms, molecules, or formula units by weighing. The above relationships allow for a number of possible conversions. Let's start with aluminum, since it provides the simplest conversion. Here are the possible conversions:. If converting from moles of aluminum to grams of aluminum, a conversion that places grams of aluminum in the numerator and moles of aluminum in the denominator would be used to enure the proper cancellation of units:. Search site Search Search. Go back to previous article.
Moles to moles calculator
This Mole Calculator finds the quantity of a substance in moles and molar mass of the substance using its chemical formula and known mass of the substance in grams. Indices should be entered as normal numbers after the appropriate elements or groups, e. H2O for a water molecule.
La sirenita 2 película completa en español latino repelis
This convention is used for most calculations involving gasses and is intended to represent conditions at sea level. Note: Always use the upper case for the first character in the element name and the lower case for the second character as in the periodic table. The quantity of substance in moles is equal to the number of molecules divided by the Avogadro constant 6. Look up the atomic mass of each atom. If you wanted to find the concentration of the hydrochloric acid, you could use our concentration calculator. In other words, the formula for molar ratio between any two elements or compounds is as simple as:. You can read our Cookie Policy here. We can leave out the word mol and not write the 1 coefficient as is our habit , so the final form of the equation, still balanced, is. Books vs e-Books Calculator. These coefficients also have the ratio check it and see , so this equation is balanced. Prepare a concept map and use the proper conversion factor. List other known quantities. Since the molar ratio helps determine how much of each substance is required to complete the reaction, you can also use it to find out which substance is the limiting reagent and which substance is in excess.
Use the mole calculator below to find the quantity of a substance using a chemical formula or known molar mass. Joe is the creator of Inch Calculator and has over 20 years of experience in engineering and construction. He holds several degrees and certifications.
It is, therefore, useful to find out exactly how many molecules of HCl are in the solution. Grab a periodic table. Use our avogadro's number calculator to understand this further. Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. Find out the molar mass of the substance hint: you can use Molar mass of the substance alone to calculate molar mass. Given: moles H 2 O Find: moles oxygen. Number of Moles? Be proud of yourself for having solved a complex problem. Molar Mass? What is molar ratio formula?

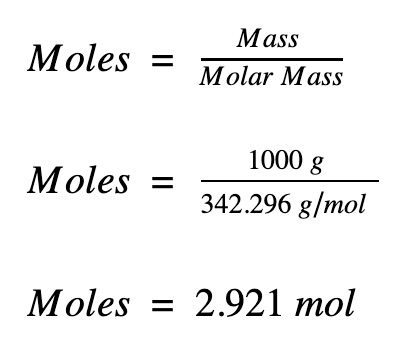
I join. And I have faced it. Let's discuss this question. Here or in PM.
Excuse for that I interfere � To me this situation is familiar. I invite to discussion. Write here or in PM.