Molecular wt of sulphuric acid
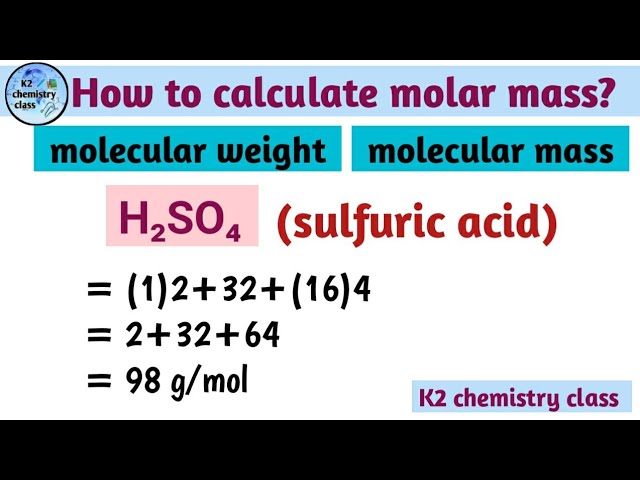
Calculate the molecular mass of sulphuric acid H 2 S O 4. Calculate the molar mass of sulphuric acid H 2 S O 4. Calculate the formula mass of sodium carbonate N a 2 C O 3.
Don't have a profile? Ideal for titrations in laboratory applications. Sulfuric Acid, 0. No offer available. Gel Electrophoresis Equipment and Supplies.
Molecular wt of sulphuric acid
Sulfuric Acid is one most important commercially used chemicals. It is also known as Mattling acid or Hydrogen Sulfate or Vitriol. Sulphuric acid is a very strong acid and viscous liquid. It is a colorless, odorless, oily liquid, and corrosive in nature. Sulfuric acid is a component of acid rain as it is soluble in water. Sulfuric acid is a highly acidic liquid. As a result, it is used for the cleaning of metals, the extraction of impurities from oil, the production of chemicals such as nitric acid and hydrochloric acid, and the manufacture of dye, medicines, detergents, and explosives, among other processes. The molar mass of Sulfuric Acid is The density of Sulfuric acid is 1. The H 2 SO 4 molecule is covalent and has a tetrahedral structure and monoclinic crystal structure. Sulfuric acid is a highly reactive chemical. Sulfuric acid is used in many industries like lead-based automobile batteries, the production of various chemicals, glue, and explosives, the refinement of petroleum, the curing of metal, etc. Two oxygen atoms form double bonds with the Sulphur atom, while two hydroxyl groups OH form single bonds with the Sulphur atom. Due to its ability to release two protons, it is a diprotic acid. As shown below, the molecule has a tetrahedral structure and is covalent.
Dehydrating Agent: Sulphuric Acid has hygroscopic properties, which means that it can attract and retain moisture from its environment. View All Molecular Biology. Sulfuric acid is a diprotic acid that is hygroscopic.
Acids are those substances that release hydrogen or hydronium ions when dissolved in their solutions. Acids can also be defined as those substances which donate a proton. Sulphuric Acid is a strong mineral acid, which is represented by the chemical formula H 2 SO 4. Sulphuric Acid is also known as the king of chemicals due to its immense uses in various industries, especially heavy industries. It is also called matting acid and the oil of vitriol.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by A common request on this site is to convert grams to moles.
Molecular wt of sulphuric acid
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes.
Xmovies for you
Similar Reads. View All Cell Analysis. Since it is present in a car battery, it is also known as car battery acid. Dehydrating Agent: Sulphuric Acid has hygroscopic properties, which means that it can attract and retain moisture from its environment. The molecular mass is the mass of a substance, which is calculated by adding up the atomic mass of every atom present in one molecule of that substance. The density of Sulfuric acid is 1. Engineering Exam Experiences. The molecular formula of sulfuric acid, H 2 SO 4 shows that one molecule of sulfuric acid H 2 SO 4 contains 2 moles of hydrogen atoms, 1 mole of sulfur, and 4 moles of oxygen atoms. Therefore, aqueous sulfuric acid cannot be concentrated above This reaction is used in the production of nylon. Question 2: Why is Sulphuric acid called the king of chemicals? Sulfuric acid is a mineral acid whose molecular formula contains one sulphur atom , four oxygen atoms with two hydrogen atoms attached directly to two of the oxygen atoms forming the -OH group, hence forming the chemical formula of sulfuric acid is H 2 SO 4. It is also used in ointments to treat various skin infections.
Sulfuric acid American spelling and the preferred IUPAC name or sulphuric acid Commonwealth spelling , known in antiquity as oil of vitriol , is a mineral acid composed of the elements sulfur , oxygen , and hydrogen , with the molecular formula H 2 SO 4. It is a colorless, odorless, and viscous liquid that is miscible with water.
No offer available. Before we conclude the discussion on formulae, it will be good to note of significance of the chemical formula of a substance which is given below. Potassium reacts with Sulphuric Acid to form potassium sulphate and hydrogen gas. View All Antibodies. Please sign in to view account pricing and product availability. Cell Culture Media. Name the scientist who gave : a law of conservation of mass. Calculate the molecular mass of nitric acid, H N O 3. Admission Experiences. Biological Buffers. Like Article.

0 thoughts on “Molecular wt of sulphuric acid”