Molecular shapes chart
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure molecular shapes chart a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity, molecular shapes chart. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules.
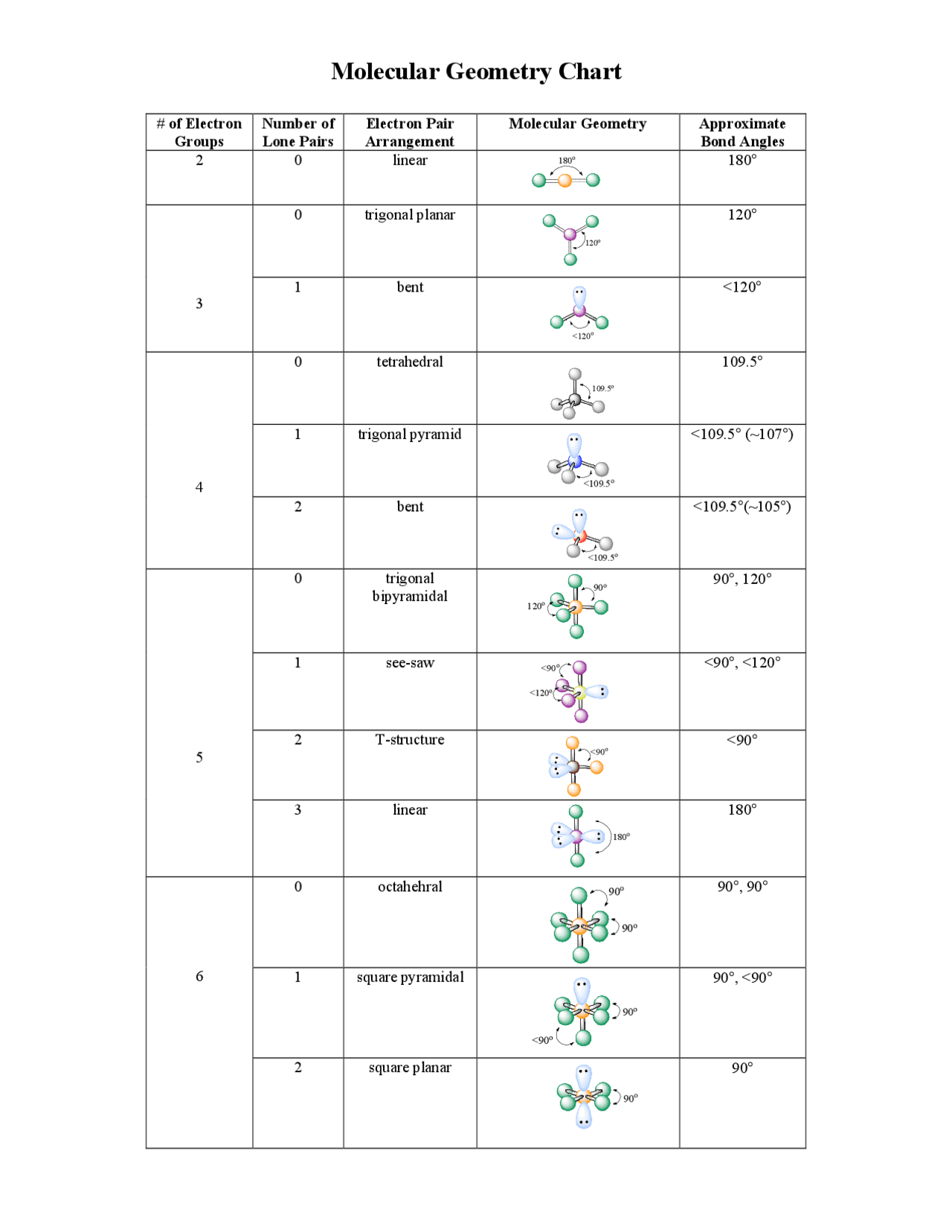
The VSEPR theory detremines molecular geometries linear, trigonal, trigonal bipyramidal, tetrahedral, and octahedral. Apply the VSEPR model to determine the geometry of a molecule that contains no lone pairs of electrons on the central atom. The valence shell electron pair repulsion VSEPR model focuses on the bonding and nonbonding electron pairs present in the outermost valence shell of an atom that connects with two or more other atoms. Fundamentally, the VSEPR model theorizes that these regions of negative electric charge will repel each other, causing them and the chemical bonds that they form to stay as far apart as possible. If the central atom also contains one or more pairs of non-bonding electrons, these additional regions of negative charge will behave much like those associated with the bonded atoms. The orbitals containing the various bonding and non-bonding pairs in the valence shell will extend out from the central atom in directions that minimize their mutual repulsions. Molecular geometries linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral are determined by the VSEPR theory.
Molecular shapes chart
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths , bond angles , torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity , polarity , phase of matter , color , magnetism and biological activity. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR , microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography , neutron diffraction and electron diffraction can give molecular structure for crystalline solids based on the distance between nuclei and concentration of electron density. Gas electron diffraction can be used for small molecules in the gas phase. NMR and FRET methods can be used to determine complementary information including relative distances, [4] [5] [6] dihedral angles, [7] [8] angles, and connectivity. Molecular geometries are best determined at low temperature because at higher temperatures the molecular structure is averaged over more accessible geometries see next section. Larger molecules often exist in multiple stable geometries conformational isomerism that are close in energy on the potential energy surface. Geometries can also be computed by ab initio quantum chemistry methods to high accuracy. The molecular geometry can be different as a solid, in solution, and as a gas. The position of each atom is determined by the nature of the chemical bonds by which it is connected to its neighboring atoms. The molecular geometry can be described by the positions of these atoms in space, evoking bond lengths of two joined atoms, bond angles of three connected atoms, and torsion angles dihedral angles of three consecutive bonds. Since the motions of the atoms in a molecule are determined by quantum mechanics, "motion" must be defined in a quantum mechanical way.
From a classical point of view it can be stated that at higher temperatures more molecules will rotate faster, which implies that they have higher angular velocity and angular momentum. Determining Polarity Is molecular shapes chart polar?
.
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths , bond angles , torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity , polarity , phase of matter , color , magnetism and biological activity. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR , microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography , neutron diffraction and electron diffraction can give molecular structure for crystalline solids based on the distance between nuclei and concentration of electron density. Gas electron diffraction can be used for small molecules in the gas phase. NMR and FRET methods can be used to determine complementary information including relative distances, [4] [5] [6] dihedral angles, [7] [8] angles, and connectivity. Molecular geometries are best determined at low temperature because at higher temperatures the molecular structure is averaged over more accessible geometries see next section. Larger molecules often exist in multiple stable geometries conformational isomerism that are close in energy on the potential energy surface.
Molecular shapes chart
The VSEPR theory detremines molecular geometries linear, trigonal, trigonal bipyramidal, tetrahedral, and octahedral. Apply the VSEPR model to determine the geometry of a molecule that contains no lone pairs of electrons on the central atom. The valence shell electron pair repulsion VSEPR model focuses on the bonding and nonbonding electron pairs present in the outermost valence shell of an atom that connects with two or more other atoms.
How to check your vo2 max on apple watch
However, we live in a 3-D world. Electron-group geometry is determined by the number of electron groups. Search site Search Search. Draw the Lewis Structure. Water has four electron groups so it falls under tetrahedral for the electron-group geometry. Let's create an analogy. B; Inverted geometries at carbon Kenneth B. Valence-Shell Electron-Pair Repulsion Theory Now that we have a background in the Lewis electron dot structure we can use it to locate the the valence electrons of the center atom. Retrieved Previous: Electronegativity. Geometries can also be computed by ab initio quantum chemistry methods to high accuracy. The electrons and the nuclei settle into positions that minimize repulsion and maximize attraction. Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
Tricapped trigonal prismatic Capped square antiprismatic. From a classical point of view it can be stated that at higher temperatures more molecules will rotate faster, which implies that they have higher angular velocity and angular momentum. So far, we have only discussed geometries without any lone pairs of electrons. If it has different terminal atoms, then it is polar. You can view a better structural formula of butane at en. Current Opinion in Structural Biology. The four equivalent bonds point in four geometrically equivalent directions in three dimensions, corresponding to the four corners of a tetrahedron centered on the carbon atom. The position of each atom is determined by the nature of the chemical bonds by which it is connected to its neighboring atoms. Authority control databases : National Germany Japan. A common example is HCl. The more electronegative end of the molecule is the negative end and the less electronegative end is the positive end. Although VSEPR theory predicts the distribution of the electrons, we have to take in consideration of the actual determinant of the molecular shape. Is it polar? Bond Angles Bond angles also contribute to the shape of a molecule.

0 thoughts on “Molecular shapes chart”